Play all audios:
ABSTRACT Kinesins are a large superfamily of molecular motors. They move along microtubule filaments and are powered by the hydrolysis of ATP. This transport system is essential for neuronal
function and survival. KIF1A belongs to the kinesin 3 family and involves in the anterograde transport of synaptic vesicle precursors along axons. Several studies confirmed that _KIF1A_
mutations cause spastic paraplegia and sensory neuropathy in an autosomal-recessive fashion. A missense mutation in the _KIF1A_ gene (p.Thr99Met) has been reported in a patient with
intellectual disability (ID), axial hypotonia and peripheral spasticity. Mild atrophy of the cerebellar vermis was found on magnetic resonance imaging. The mutation was heterozygous and _de
novo_. We identified the second patient with the p.T99M mutation in the _KIF1A_ gene by whole-exome sequencing. He showed severe ID, spasticity, optic atrophy, neurogenic bladder, growth
failure and progressive cerebellar atrophy. The p.T99M mutation may be a common recurrent mutation. We suppose that this specific mutation of _KIF1A_ shows a novel neurodegenerative
syndrome. SIMILAR CONTENT BEING VIEWED BY OTHERS FURTHER DELINEATION OF _KIF21B-_RELATED NEURODEVELOPMENTAL DISORDERS Article 06 October 2022 NOVEL _CWF19L1_ MUTATIONS IN PATIENTS WITH
SPINOCEREBELLAR ATAXIA, AUTOSOMAL RECESSIVE 17 Article 26 September 2023 ALTERED MOLECULAR AND CELLULAR MECHANISMS IN _KIF5A_-ASSOCIATED NEURODEGENERATIVE OR NEURODEVELOPMENTAL DISORDERS
Article Open access 27 September 2024 INTRODUCTION Kinesins are a large superfamily of molecular motors. Kinesins move along microtubule filaments and are powered by the hydrolysis of ATP.
_KIF1A_ (MIM*601255) encodes a motor protein gene involved in the anterograde transport of synaptic vesicle precursors along axons.1 Knockout of _Kif1a_ in mice is lethal soon after birth
due to massive axonal and neuronal cell body degeneration in the central nervous system.2 KIF1A transports a synaptic vesicle precursor and KIF1A-mediated axonal transport has a critical
role in the viability, maintenance and function of neurons, particularly mature neurons.2 Abnormalities in the axonal transport of synaptic vesicles due to _KIF1A_ mutations cause spastic
paraplegia and sensory neuropathy in an autosomal-recessive fashion.3, 4, 5 Hamdan _et al._6 identified a missense mutation (p.Thr99Met) in the _KIF1A_ gene with whole-exome sequencing (WES)
in a patient with non-syndrome intellectual disability (ID). The mutation was heterozygous and _de novo_. We identified the second patient with the p.Thr99Met mutation. MATERIALS AND
METHODS With the approval of our institutional ethics committee, we planned a proband-parent trio approach using WES (Supplementary Methods). We generated 6.54 Gb sequence on average. The
average read depth of the on-target regions was 53.4. To check the quality of our WES base calls, we genotyped eight samples using both next-generation sequencing (NGS) and single nucleotide
polymorphism (SNP) array and compared them. On average, the genotypes for 244 698 locations were comparable between the two data sets and 99.95% genotypes were concordant between our NGS
calls and the genotype of the SNP array (Supplementary Table S1). We validated the 140 mismatched positions for the NA18943 sample by SureSelect V4 (Agilent Technologies, Santa Clara, CA,
USA) that were discordant using a Sanger sequencing (Supplementary Tables S2 and S3). As a result, 77 of 140 genotypes were consistent with our NGS calls. On the basis of this validation, we
estimated the false positive and false negative rates of our calling methods to be 0.021% (50/243 573) and 0.064% (13/20 424), respectively. Note that these rates are conservative
estimations, as the single nucleotide variants (SNVs) that could not be successfully determined using Sanger sequencing were counted as mismatched. In addition, the test sample, NA18943, had
the most discordant SNP calls of the set. RESULTS CLINICAL REPORT The 8-year-old boy was the third child of healthy and non-consanguineous Japanese parents. After an uncomplicated
pregnancy, he was born at 40 weeks of gestation. His birth weight was 3700 g, length was 51 cm and head circumference was 34 cm. He was hypotonic and his developmental milestones were
markedly delayed. Routine laboratory tests revealed mildly elevated transaminases. Aspartate aminotransferase ranged from 77 to 170 IU l−1 (normal <30 IU l−1), whereas alanine
transaminase ranged from 58 to 206 IU l−1 (normal <30 IU l−1) during infantile period. G-banded chromosome analysis was normal. Brain magnetic resonance imaging at 8 months of age
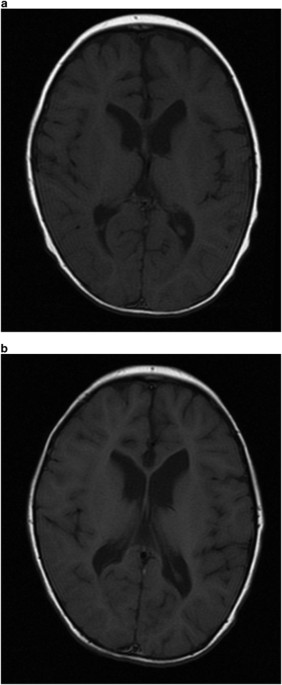
revealed mildly enlarged lateral ventricles, hypoplasia of the corpus callosum, thin pituitary gland and atrophic cerebellar vermis (Figures 1 and 2). Physical examination identified no
dysmorphic features. He showed axial hypotonia and peripheral spasticity. Deep tendon reflexes were exaggerated. Nystagmus was noted. Ocular pursuit was poor. Ophthalmological investigation
revealed optic atrophy. Visual evoked potentials showed a poor response. Head control was achieved at 18 months of age. He rolled over at 2 years of age and could sit without support at 3
years of age. His height gain was under −3 s.d., and complete isolated growth hormone deficiency was revealed. Replacement therapy was started from 3 years of age. Muscle biopsy gave
nonspecific findings. Electromyograms with nerve conduction velocity studies revealed normal findings. Generalized tonic-clonic convulsion appeared at 4 years of age. Electroencephalography
(EEG) showed diffuse small spikes. His locomotive activity gradually decreased. Axial hypotonia and peripheral spasticity became more apparent. His lower extremities were spastic and his
left hip joint was dislocated. His height was 106 cm (−1.6 s.d.) and weight was 14.7 kg (−1.9 s.d.). His head circumference was 47.5 cm (−2.4 s.d.) at 6 years of age. He had no cognitive and
language perception. Obstructive sleep apnea appeared. He developed repeated episodes of urinary retention. Ultrasonography of the urinary tract showed a distended bladder with normal
kidneys and he was diagnosed with neurogenic bladder. He was started on clean intermittent catheterization to ensure adequate bladder drainage. He also showed chronic constipation. Repeated
neuroradiological investigations showed progressive cerebral and cerebellar atrophy (Figures 1 and 2). WHOLE-EXOME SEQUENCING A _de novo_ missense mutation,
NM_001244008:exon4:c.C296T:p.T99M, was identified in _KIF1A_. This mutation was confirmed by Sanger sequencing. DISCUSSION Hamdan _et al._6 reported a patient with p.T99M mutation in the
_KIF1A_ gene. The patient was a 3-year-old girl with severe ID, axial hypotonia and peripheral spasticity. Mild atrophy of the vermian region of the cerebellum was found. The patient showed
similar conditions to our patient. Our patient showed progressive cerebral and cerebellar atrophy. The progressive nature of _KIF1A_ mutations can be explained by the evidence that mature
neurons are particularly affected.2 Optic atrophy and neurogenic bladder were novel findings in our patient. We supposed that p.T99M mutation may affect optic nerve and autonomic nervous
system. Complete growth hormone (GH) deficiency might be a coincidental complication. Hamdan _et al._6 studied the impact of p.T99M on KIF1A subcellular localization in neurites. They
transfected primary rat hippocampal neurons with KIF1A motor domain–EGFP fusion constructs. Wild-type KIF1A accumulated in distal regions of neurites, whereas KIF1A molecule with the p.T99M
mutation showed greatly reduced distal localization and increased accumulation throughout the cell body and proximal neurites.6 Because the T99 residue lies in the conserved ATP-binding p
loop of the motor domain of KIF1A, the mutation may affect KIF1A to obtain energy from ATP. As KIF1A can homodimerize, heterozygous mutations may cause a dominant negative effect. It is
interesting to investigate patients with neurodegeration of unknown cause for p.T99M mutation, which may be a common recurrent mutation. We propose that this specific mutation of _KIF1A_
shows a novel neurodegenerative syndrome. REFERENCES * Okada Y ., Yamazaki H ., Sekine-Aizawa Y ., Hirokawa N . The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric
motor for anterograde axonaltransport of synaptic vesicle precursors. _Cell_ 1995; 81: 769–780. Article CAS Google Scholar * Yonekawa Y ., Harada A ., Okada Y ., Funakoshi T ., Kanai Y .,
Takei Y . _et al_. Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. _J. Cell Biol._ 1998; 141: 431–441. Article CAS Google
Scholar * Erlich Y ., Edvardson S ., Hodges E ., Zenvirt S ., Thekkat P ., Shaag A . _et al_. Exome sequencing and disease-network analysis of a single family implicate a mutation in KIF1A
in hereditary spastic paraparesis. _Genome Res._ 2011; 21: 658–664. Article CAS Google Scholar * Rivière J. B ., Ramalingam S ., Lavastre V ., Shekarabi M ., Holbert S ., Lafontaine J .
_et al_. KIF1A, an axonal transporter of synaptic vesicles, is mutated in hereditary sensory and autonomic neuropathy type 2. _Am. J. Hum. Genet._ 2011; 89: 219–230. Article Google Scholar
* Klebe S ., Lossos A ., Azzedine H ., Mundwiller E ., Sheffer R ., Gaussen M . _et al_. KIF1A missense mutations in SPG30, an autosomal recessive spastic paraplegia: distinct phenotypes
according to the nature of the mutations. _Eur. J. Hum. Genet._ 2012; 20: 645–649. Article CAS Google Scholar * Hamdan F. F ., Gauthier J ., Araki Y ., Lin D. T ., Yoshizawa Y ., Higashi
K . _et al_. Excess of de novo deleterious mutations in genes associated with glutamatergic systems in nonsyndromic intellectual disability. _Am. J. Hum. Genet._ 2011; 88: 306–316. Article
CAS Google Scholar Download references ACKNOWLEDGEMENTS This study was supported by the Health and Labour Research Grants in 2013 by Ministry of Health, Labour and welfare in Japan. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Department of Medical Genetics, Osaka Medical Center and Research Institute for Maternal and Child Health, Osaka, Japan Nobuhiko Okamoto * Laboratory
for Medical Science Mathematics, Center for Integrative Medical Sciences, RIKEN, Yokohama, Japan Fuyuki Miya & Tatsuhiko Tsunoda * Department of Pediatric Neurology, Osaka Medical Center
and Research Institute for Maternal and Child Health, Osaka, Japan Keiko Yanagihara * Department of Pediatrics, Yamagata University Faculty of Medicine, Yamagata, Japan Mitsuhiro Kato *
Department of Pediatrics and Neonatology, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan Shinji Saitoh * Department of Pediatric Neurosurgery, Takatsuki General
Hospital, Osaka, Japan Mami Yamasaki * Division of Regenerative Medicine, Institute for Clinical Research, Osaka National Hospital, National Hospital Organization, Osaka, Japan Yonehiro
Kanemura * Department of Neurosurgery, Osaka National Hospital, National Hospital Organization, Osaka, Japan Yonehiro Kanemura * Center for Medical Genetics, Keio University School of
Medicine, Tokyo, Japan Kenjiro Kosaki Authors * Nobuhiko Okamoto View author publications You can also search for this author inPubMed Google Scholar * Fuyuki Miya View author publications
You can also search for this author inPubMed Google Scholar * Tatsuhiko Tsunoda View author publications You can also search for this author inPubMed Google Scholar * Keiko Yanagihara View
author publications You can also search for this author inPubMed Google Scholar * Mitsuhiro Kato View author publications You can also search for this author inPubMed Google Scholar * Shinji
Saitoh View author publications You can also search for this author inPubMed Google Scholar * Mami Yamasaki View author publications You can also search for this author inPubMed Google
Scholar * Yonehiro Kanemura View author publications You can also search for this author inPubMed Google Scholar * Kenjiro Kosaki View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Nobuhiko Okamoto. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL INFORMATION
Supplementary Information accompanies the paper on Journal of Human Genetics website SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION (DOC 31 KB) SUPPLEMENTARY TABLE S1 (PDF 80 KB)
SUPPLEMENTARY TABLE S3 (PDF 70 KB) SUPPLEMENTARY TABLE S2 (XLS 92 KB) RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Okamoto, N., Miya, F., Tsunoda, T.
_et al._ _KIF1A_ mutation in a patient with progressive neurodegeneration. _J Hum Genet_ 59, 639–641 (2014). https://doi.org/10.1038/jhg.2014.80 Download citation * Received: 15 June 2014 *
Revised: 01 August 2014 * Accepted: 27 August 2014 * Published: 25 September 2014 * Issue Date: November 2014 * DOI: https://doi.org/10.1038/jhg.2014.80 SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative