Play all audios:
ABSTRACT Epigenetic mechanisms that maintain neurogenesis throughout adult life remain poorly understood1. Trithorax group (trxG) and Polycomb group (PcG) gene products are part of an
evolutionarily conserved chromatin remodelling system that activate or silence gene expression, respectively2. Although PcG member _Bmi1_ has been shown to be required for postnatal neural
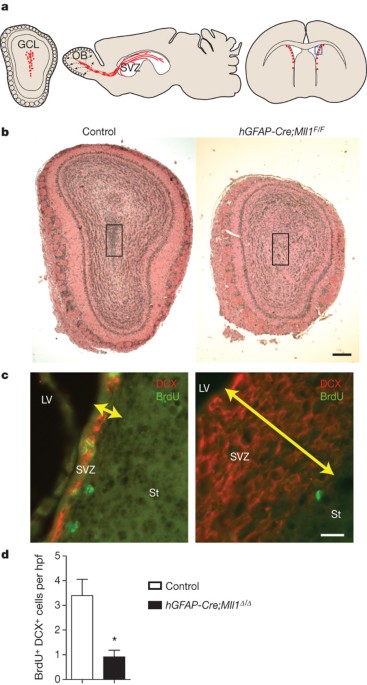
stem cell self-renewal3,4, the role of trxG genes remains unknown. Here we show that the trxG member _Mll1_ (_mixed-lineage leukaemia 1_) is required for neurogenesis in the mouse postnatal
brain. _Mll1_-deficient subventricular zone neural stem cells survive, proliferate and efficiently differentiate into glial lineages; however, neuronal differentiation is severely impaired.
In _Mll1_-deficient cells, early proneural _Mash1_ (also known as Ascl1) and gliogenic _Olig2_ expression are preserved, but _Dlx2_, a key downstream regulator of subventricular zone
neurogenesis, is not expressed. Overexpression of _Dlx2_ can rescue neurogenesis in _Mll1_-deficient cells. Chromatin immunoprecipitation demonstrates that _Dlx2_ is a direct target of MLL
in subventricular zone cells. In differentiating wild-type subventricular zone cells, _Mash1_, _Olig2_ and _Dlx2_ loci have high levels of histone 3 trimethylated at lysine 4 (H3K4me3),
consistent with their transcription. In contrast, in _Mll1_-deficient subventricular zone cells, chromatin at _Dlx2_ is bivalently marked by both H3K4me3 and histone 3 trimethylated at
lysine 27 (H3K27me3), and the _Dlx2_ gene fails to properly activate. These data support a model in which _Mll1_ is required to resolve key silenced bivalent loci in postnatal neural
precursors to the actively transcribed state for the induction of neurogenesis, but not for gliogenesis. Access through your institution Buy or subscribe This is a preview of subscription
content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS YBX1 FINE-TUNES PRC2 ACTIVITIES TO
CONTROL EMBRYONIC BRAIN DEVELOPMENT Article Open access 13 August 2020 ZFP462 SAFEGUARDS NEURAL LINEAGE SPECIFICATION BY TARGETING G9A/GLP-MEDIATED HETEROCHROMATIN TO SILENCE ENHANCERS
Article 05 January 2023 ASH2L REGULATES POSTNATAL NEUROGENESIS THROUGH ONECUT2-MEDIATED INHIBITION OF TGF-Β SIGNALING PATHWAY Article 11 July 2023 REFERENCES * Hsieh, J. & Gage, F. H.
Epigenetic control of neural stem cell fate. _Curr. Opin. Genet. Dev._ 14, 461–469 (2004) Article CAS Google Scholar * Schuettengruber, B., Chourrout, D., Vervoort, M., Leblanc, B. &
Cavalli, G. Genome regulation by polycomb and trithorax proteins. _Cell_ 128, 735–745 (2007) Article CAS Google Scholar * Molofsky, A. V. et al. Bmi-1 dependence distinguishes neural stem
cell self-renewal from progenitor proliferation. _Nature_ 425, 962–967 (2003) Article ADS CAS Google Scholar * Fasano, C. A. et al. shRNA knockdown of _Bmi-1_ reveals a critical role
for p21-Rb pathway in NSC self-renewal during development. _Cell Stem Cell_ 1, 87–99 (2007) Article CAS Google Scholar * Lim, D. A. et al. _In vivo_ transcriptional profile analysis
reveals RNA splicing and chromatin remodeling as prominent processes for adult neurogenesis. _Mol. Cell. Neurosci._ 31, 131–148 (2006) Article CAS Google Scholar * Daser, A. &
Rabbitts, T. H. Extending the repertoire of the mixed-lineage leukemia gene _MLL_ in leukemogenesis. _Genes Dev._ 18, 965–974 (2004) Article CAS Google Scholar * Yu, B. D., Hess, J. L.,
Horning, S. E., Brown, G. A. & Korsmeyer, S. J. Altered Hox expression and segmental identity in _Mll_-mutant mice. _Nature_ 378, 505–508 (1995) Article ADS CAS Google Scholar *
Jude, C. D. et al. Unique and independent roles for MLL in adult hematopoietic stem cells and progenitors. _Cell Stem Cell_ 1, 324–337 (2007) Article CAS Google Scholar * Zhuo, L. et al.
hGFAP-cre transgenic mice for manipulation of glial and neuronal function _in vivo_ . _Genesis_ 31, 85–94 (2001) Article CAS Google Scholar * Han, Y. G. et al. Hedgehog signaling and
primary cilia are required for the formation of adult neural stem cells. _Nature Neurosci._ 11, 277–284 (2008) Article CAS Google Scholar * Luskin, M. B. Restricted proliferation and
migration of postnatally generated neurons derived from the forebrain subventricular zone. _Neuron_ 11, 173–189 (1993) Article CAS Google Scholar * Lois, C. & Alvarez-Buylla, A.
Long-distance neuronal migration in the adult mammalian brain. _Science_ 264, 1145–1148 (1994) Article ADS CAS Google Scholar * Doetsch, F., Caille, I., Lim, D. A., Garcia-Verdugo, J. M.
& Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. _Cell_ 97, 703–716 (1999) Article CAS Google Scholar * Wichterle, H.,
Garcia-Verdugo, J. M. & Alvarez-Buylla, A. Direct evidence for homotypic, glia-independent neuronal migration. _Neuron_ 18, 779–791 (1997) Article CAS Google Scholar * Marshall, C.
A., Suzuki, S. O. & Goldman, J. E. Gliogenic and neurogenic progenitors of the subventricular zone: who are they, where did they come from, and where are they going? _Glia_ 43, 52–61
(2003) Article Google Scholar * Picard-Riera, N. et al. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in
adult mice. _Proc. Natl Acad. Sci. USA_ 99, 13211–13216 (2002) Article ADS CAS Google Scholar * Menn, B. et al. Origin of oligodendrocytes in the subventricular zone of the adult brain.
_J. Neurosci._ 26, 7907–7918 (2006) Article CAS Google Scholar * Spassky, N. et al. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis.
_J. Neurosci._ 25, 10–18 (2005) Article CAS Google Scholar * Lu, Q. R. et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection.
_Cell_ 109, 75–86 (2002) Article CAS Google Scholar * Zhou, Q. & Anderson, D. J. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. _Cell_
109, 61–73 (2002) Article CAS Google Scholar * Scheffler, B. et al. Phenotypic and functional characterization of adult brain neuropoiesis. _Proc. Natl Acad. Sci. USA_ 102, 9353–9358
(2005) Article ADS CAS Google Scholar * Novak, A., Guo, C., Yang, W., Nagy, A. & Lobe, C. G. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon
Cre-mediated excision. _Genesis_ 28, 147–155 (2000) Article CAS Google Scholar * Zappone, M. V. et al. Sox2 regulatory sequences direct expression of a β-geo transgene to telencephalic
neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. _Development_ 127, 2367–2382 (2000) CAS PubMed Google Scholar *
Parras, C. M. et al. _Mash1_ specifies neurons and oligodendrocytes in the postnatal brain. _EMBO J._ 23, 4495–4505 (2004) Article CAS Google Scholar * Long, J. E. et al. Dlx-dependent
and -independent regulation of olfactory bulb interneuron differentiation. _J. Neurosci._ 27, 3230–3243 (2007) Article CAS Google Scholar * Bernstein, B. E. et al. A bivalent chromatin
structure marks key developmental genes in embryonic stem cells. _Cell_ 125, 315–326 (2006) Article CAS Google Scholar * Milne, T. A. et al. MLL targets SET domain methyltransferase
activity to _Hox_ gene promoters. _Mol. Cell_ 10, 1107–1117 (2002) Article CAS Google Scholar * Lee, M. G. et al. Demethylation of H3K27 regulates polycomb recruitment and H2A
ubiquitination. _Science_ 318, 447–450 (2007) Article ADS CAS Google Scholar * Swigut, T. & Wysocka, J. H3K27 demethylases, at long last. _Cell_ 131, 29–32 (2007) Article CAS
Google Scholar * Mikkelsen, T. S. et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. _Nature_ 448, 553–560 (2007) Article ADS CAS Google Scholar *
Wysocka, J. et al. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. _Cell_ 121, 859–872 (2005) Article CAS Google Scholar
Download references ACKNOWLEDGEMENTS We thank J. Rubenstein for anti-DLX2 antibodies and the pCAG-Dlx2 plasmid, D. Rowitch for anti-OLIG2 antibodies, and Y. Dou and R. Roeder for anti-MLL1
antibodies. This work was supported by the Neurosurgery Research and Education Foundation/American Association of Neurological Surgeons, Sandler Family Foundation, Northern California
Institute for Research and Education, and the Clinical and Translational Research Institute at the University of California, San Francisco (D.A.L.), California Institute for Regenerative
Medicine New Faculty Award and The Chicago Community Trust Searle Scholar Award (J.W.), and the Goldhirsch Foundation, J.G. Bowes Research Fund, and National Institutes of Health (NIH)
5R37-NS028478 (A.A.-B.). AUTHOR CONTRIBUTIONS D.A.L. conceived the project, designed and performed experiments, coordinated collaborations, and wrote the manuscript. Y.-C.H. worked on most
experiments, quantified all _in vivo_ data, and helped prepare the figures. T.S. and J.W. performed ChIP experiments, helped analyse data and contributed ideas. A.L.M and P.A.E. provided the
_Mll1__F/F_ mouse, helped perform preliminary experiments in _Mll1_+/- mice and contributed ideas. J.M.G.V. provided electron microscopy data and histological interpretation. A.A.-B.
contributed ideas, interpreted results and helped write the manuscript. All authors discussed the results and edited the manuscript. AUTHOR INFORMATION Author notes * Yin-Cheng Huang Present
address: Present address: Department of Neurosurgery, Graduate Institute of Clinical Medical Science, ChangGung Univerisity, Kwei-Shan, Tao-yuan, Taiwan., * Daniel A. Lim and Yin-Cheng
Huang: These authors contributed equally to this work. AUTHORS AND AFFILIATIONS * Department of Neurological Surgery,, Daniel A. Lim, Yin-Cheng Huang & Arturo Alvarez-Buylla * Institute
for Regeneration Medicine, and,, Daniel A. Lim, Yin-Cheng Huang & Arturo Alvarez-Buylla * Veteran’s Affairs Medical Center, University of California, San Francisco, 505 Parnassus Street
M779, San Francisco, California 94143, USA , Daniel A. Lim * Department of Chemical and Systems Biology, Department of Developmental Biology Stanford University School of Medicine, Stanford,
California 94305, USA, Tomek Swigut & Joanna Wysocka * Department of Genetics, Dartmouth Medical School, Hanover, New Hampshire 03755, USA, Anika L. Mirick & Patricia Ernst *
Laboratorio de Neurobiologia Comparada, Instituto Cavanilles, Universidad de Valencia, Valencia 46012, Spain Jose Manuel Garcia-Verdugo * Laboratorio de Morfologia Celular, Centro de
Investigación Príncipe Felipe, CIBERNED, Valencia 46012, Spain , Jose Manuel Garcia-Verdugo Authors * Daniel A. Lim View author publications You can also search for this author inPubMed
Google Scholar * Yin-Cheng Huang View author publications You can also search for this author inPubMed Google Scholar * Tomek Swigut View author publications You can also search for this
author inPubMed Google Scholar * Anika L. Mirick View author publications You can also search for this author inPubMed Google Scholar * Jose Manuel Garcia-Verdugo View author publications
You can also search for this author inPubMed Google Scholar * Joanna Wysocka View author publications You can also search for this author inPubMed Google Scholar * Patricia Ernst View author
publications You can also search for this author inPubMed Google Scholar * Arturo Alvarez-Buylla View author publications You can also search for this author inPubMed Google Scholar
CORRESPONDING AUTHORS Correspondence to Daniel A. Lim or Arturo Alvarez-Buylla. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES This file contains Supplementary Figures 1-9 with Legends (PDF
4861 kb) POWERPOINT SLIDES POWERPOINT SLIDE FOR FIG. 1 POWERPOINT SLIDE FOR FIG. 2 POWERPOINT SLIDE FOR FIG. 3 POWERPOINT SLIDE FOR FIG. 4 RIGHTS AND PERMISSIONS Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Lim, D., Huang, YC., Swigut, T. _et al._ Chromatin remodelling factor _Mll1_ is essential for neurogenesis from postnatal neural stem cells. _Nature_
458, 529–533 (2009). https://doi.org/10.1038/nature07726 Download citation * Received: 23 July 2007 * Accepted: 15 December 2008 * Published: 11 February 2009 * Issue Date: 26 March 2009 *
DOI: https://doi.org/10.1038/nature07726 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative