Play all audios:
ABSTRACT OBJECTIVE The Downes score is a neonatal examination scoring tool frequently used to guide initiation of CPAP, but its ability to predict the need for surfactant has not been
assessed. We assessed the extent to which the Downes score predicts the receipt of surfactant. STUDY DESIGN We calculated a simplified Downes score from nursing admission data for infants (≤
2000 grams, and ≥ 25 weeks’ gestation) admitted on CPAP to a highly resourced level III NICU, to assess the predictive value for the receipt of surfactant. RESULTS Fifty-three (31.5%) out
of 168 infants admitted on CPAP received surfactant. A simplified Downes score of ≥ 4 predicted the receipt of surfactant with 90.6% sensitivity, 52.2% specificity, 46.6% positive predictive
value, 92.3% negative predictive value, and 64.3% accuracy. CONCLUSION The high sensitivity and negative predictive value suggest utility for using the Downes score to help guide clinical
decision making regarding surfactant therapy. SIMILAR CONTENT BEING VIEWED BY OTHERS LUNG ULTRASOUND SCORE FOR PREDICTION OF SURFACTANT ADMINISTRATION IN PRETERM INFANTS WITH RESPIRATORY
FAILURE Article 09 August 2024 A SIMPLE SCORING SYSTEM FOR PREDICTION OF IVH IN VERY-LOW-BIRTH-WEIGHT INFANTS Article 21 July 2023 MACHINE LEARNING TO PREDICT LATE RESPIRATORY SUPPORT IN
PRETERM INFANTS: A RETROSPECTIVE COHORT STUDY Article Open access 17 February 2023 BACKGROUND Respiratory distress syndrome (RDS) is a major cause of death amongst preterm neonates
worldwide, and a significant contributor to complications associated with preterm birth [1, 2]. The most common supportive treatments for RDS include supplemental oxygen and continuous
positive airway pressure (CPAP) [3]. While most infants can be successfully managed on CPAP, the requirement for invasive mechanical ventilation (IMV), or CPAP failure, occurs in
approximately 20–40% of preterm infants [4, 5]. CPAP failure has been reported to be higher in areas with lower antenatal corticosteroid administration [6]. Early selective surfactant
administration in infants at risk of CPAP failure can help prevent IMV and complications associated with its use [7,8,9]. Early identification of neonates at risk of CPAP failure is not only
necessary in highly resourced settings that can provide surfactant and/or mechanical ventilation, but is also important in lower-resourced settings when transfer to a higher level of care
with advanced respiratory support is feasible [1]. There are various methods used to identify infants that would most benefit from surfactant administration, including physiologic measures
[Fraction of inspired oxygen (FiO2), respiratory severity score or mean airway pressure (MAP): FiO2 product], blood gas parameters (pCO2 and pH), and lung imaging (lung ultrasound, and chest
radiograph) [9,10,11,12]. Although a FiO2 of 0.3 to 0.4 has been the most frequently used parameter in practice and in many studies, the optimal threshold remains unknown and likely varies
based on gestational age [9, 13,14,15,16]. Clinical prediction scores have also been developed, but many incorporate gestational age or other factors that may be unknown or difficult to
obtain in low-resource settings [17]. The Downes score was originally developed in 1970. Although its ability to predict the need for surfactant administration was not studied, it is being
used extensively in low-resourced settings to objectively measure respiratory distress, help guide CPAP therapy, and predict CPAP failure [18,19,20,21,22,23,24,25], likely because it
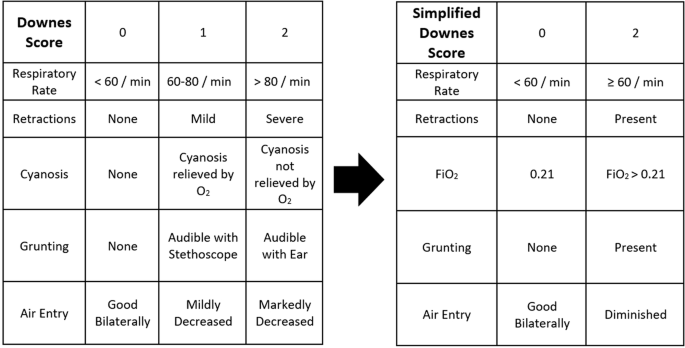
correlates well with physiological variables reflecting severe RDS [18]. The Downes score includes cyanosis (and whether or not it is relieved by supplemental FiO2), respiratory rate,
retractions, grunting, and decreased air entry on auscultation. Each category is given 0, 1, or 2 points which is aggregated into a total score of 0 to 10. Scores of 4 and greater have been
commonly used to guide respiratory support and predict CPAP failure in low-resource settings [21,22,23, 25]. The recently published RDS-NeXT Workshop consensus statements recommended the use
of the modified Downes score as a clinical examination scoring tool in addition to FiO2, level of respiratory support, and blood gas parameters (pH or pCO2), if available, to help guide
surfactant administration [26]. The aim of this study was to determine if a simplified Downes score obtained from nursing admission data for infants admitted on nasal CPAP would be able to
identify, with high sensitivity and specificity, the infants that received surfactant therapy in a highly resourced level III NICU. PATIENTS AND METHODS This study was an analysis of
existing nursing admission data for infants admitted on nasal CPAP. The primary objective was to evaluate the predictive value of a simplified Downes score in the first hour of life for the
receipt of surfactant, with or without mechanical ventilation. The secondary objective was to investigate the predictive value of individual components of the simplified Downes score (FiO2
> 0.21, respiratory rate ≥60, retractions, grunting, decreased air entry on auscultation) for the receipt of surfactant, with and without mechanical ventilation. The University of Vermont
Institutional Review Board determined that this study was not human subjects research. Epic SlicerDicer was used to search the University of Vermont Medical Center (UVMMC) Level III NICU
electronic medical record (EMR) for all infants admitted on CPAP between January 1, 2018 and January 31, 2023 who weighed ≤ 2000 grams and were ≥ 25 weeks’ gestation at birth. The weight
cutoff was selected with the goal of identifying patients who may be considered preterm based on birthweight alone, in the absence of accurate gestational age dating, such that may occur in
lower-resourced settings. The gestational age cutoff was chosen because our institution had protocolized routine delivery room intubation in infants <25 weeks’ gestation during the
selected time period. At UVMMC, nasal bubble CPAP is commonly initiated at 5–6 cm H2O. For infants receiving surfactant, the practice during the study period was to provide an initial dose
of 2.5 mL/kg of CUROSURF® (poractant alfa) via the INSURE (intubate-surfactant-extubate) technique, or endotracheal tube (ETT) if intubated for continued IMV. For infants to be designated as
having received INSURE in this study, they were not placed on the ventilator and did not have radiographic confirmation of ETT placement. Although a FiO2 > 0.3 is a common threshold for
surfactant administration at our institution, clinician discretion allows deviation from this guideline to individualize care in the best interest of patients. For infants identified from
the EMR, chart review was performed starting with exclusionary criteria. Infants were excluded if they were outborn, intubated in the delivery room, or had severe congenital anomalies known
prenatally or identified on admission. For infants fulfilling the inclusion criteria, nursing admission flowsheet data was searched for a documented admission physical examination within the
first hour of life. Infants without a documented admission physical examination were excluded. In patients for whom there was a documented admission physical examination from nursing
admission flowsheet data, the simplified Downes score was calculated using FiO2, respiratory rate, and the documented presence of retractions, grunting, and decreased air entry on
auscultation (Fig. 1). UVMMC does not currently use the Downes score in clinical care, and nursing electronic flowsheet data does not routinely discriminate between the severity of grunting
(audible by ear versus audible with auscultation), retractions (mild versus severe), or degree of air entry. For this reason, and to obtain information from nursing admission data, a
simplified Downes score was designed. The Downes score was simplified by: (1) removing the distinction between mild and severe for the presence of retractions, grunting, and decreased air
entry; (2) not discriminating between tachypnea ≥ 60 and > 80; and (3) using any FiO2 > 0.21 instead of the presence of cyanosis. The simplified Downes score removed the “middle” score
of a 1, so infants could only obtain a score of 0 or 2 in each category (Fig. 1). Setty and colleagues simplified the Silverman-Andersen score in a similar way to help facilitate use in
low-resourced settings [27]. A documented presence of the possible predictors (grunting, retractions, and decreased air entry) was counted as a “true” positive and used to calculate the
simplified Downes score. For possible predictors that were not documented in the nursing admission data flowsheet in the first hour of life (grunting, retractions, and decreased air entry),
other note types (admission history and physical, nursing note, respiratory therapy note) were searched for the presence of possible predictors to determine if they were truly absent,
equating to a “true” negative or simply missing/incomplete. If discordance was found (documented presence found in other note type, with no mention in the nursing admission data flowsheet
for ≥2 out of 3 variables), the patient was excluded from the study due to incomplete data. If there was concordance found (no documented presence found in other note type, with no mention
in the nursing admission data flowsheet for ≥2 out of 3 variables), then the possible predictors were to be considered true negatives and the patient would be included in the study. For all
patients included in the study, demographic and clinical data were also recorded. To assess the validity and feasibility of using nursing admission documentation to calculate the simplified
Downes score, we performed an initial assessment of 20 patient charts using the above assessment for discordance in documentation. A preplanned study stopping threshold was set, where if 25%
of charts (5 out of 20) had discordance between admission documentation assessments, the study would be terminated. Due to finding < 20% discordance among the infants in the initial
chart review, the study was continued as planned. Additionally, there was no evidence that gestational age (either higher or lower) contributed to discordance. The sensitivity, specificity,
positive predictive value (PPV), negative predictive value (NPV), and accuracy were calculated for both the total simplified Downes score and its individual components for the receipt of
surfactant. We also calculated receiver operating characteristic (ROC) curves and the area under the receiver (AUC) operating characteristic curve statistic. SAS 9.4 (Cary, North Carolina,
USA) was used for all analyses. RESULTS During the study period, 202 infants were admitted on CPAP. Of these 202 infants, 34 infants were excluded due to discordance or incomplete
documentation. A simplified Downes score was calculated from nursing admission flowsheet data for 168 infants (final cohort). From the final cohort, 53 (31.5%) infants received surfactant.
Demographic and clinical data are presented in Table 1. Five out of 168 infants (2.9%) were greater than 37 weeks’ gestation. A simplified Downes score of ≥4 predicted the receipt of
surfactant with a sensitivity of 90.6%, specificity of 52.2%, PPV of 46.6%, NPV of 92.3%, and accuracy of 64.3% (Table 2). The predictive value of the individual components of the simplified
Downes score are displayed in Table 2. Diminished breath sounds by itself had good accuracy at 71.4%. FiO2 by itself showed high sensitivity (81.1%) and NPV (87%), but was not very specific
(58.3%). Accuracy of FiO2 on admission was 65.5% for predicting the receipt of surfactant. As the simplified Downes score increased, the likelihood of receiving surfactant increased (Fig.
2). The ROC curves are shown in Fig. 3. The AUC was 0.78, but improved to 0.83 after adjusting for gestational age. Of the final cohort, 19.6% of infants admitted on CPAP received escalated
respiratory support with invasive mechanical ventilation in the first 72 h of life. This included infants who were intubated for surfactant (not INSURE), intubated for respiratory failure
(and did not receive surfactant), and those intubated after initially receiving INSURE. DISCUSSION When using the simplified Downes score as an objective screening tool on admission, a score
of ≥ 4 demonstrated both high sensitivity and NPV for the receipt of surfactant. The recent RDS-NeXT Workshop consensus statements, designed to help guide surfactant therapy in highly
resourced settings, included the Downes score as an objective measure of respiratory distress [26]. Although the Downes score is commonly used in low-resourced settings to help guide
respiratory support, to the best of our knowledge, this is the first publication with the aim of studying the ability of the Downes score to predict the need for surfactant. The results of
this study suggest utility in using a reliable tool such as the Downes score, or a simplified version as used in this study, to help predict which infants require surfactant. When applying a
threshold of 4 or greater, the high NPV of the simplified Downes score could be useful to help reassure practitioners that infants with low scores are less likely to require surfactant.
This finding is in line with Downes’ original description where the observation was made that “infants whose score remained less than 4 throughout the first eight hours of life were nearly
always normal from a clinical standpoint by age 24 h” [18]. At the higher cutoff of 8, the specificity and PPV of the simplified Downes score were very good at predicting the receipt of
surfactant. Not only do these results suggest the benefits of using a simple and reliable tool to help improve the early identification of infants who may require surfactant therapy in
highly resourced settings, but there will likely be application as well in translating the results back to low-resourced settings where the score is already being used. The ability of FiO2
alone to predict CPAP failure and surfactant receipt is complicated, as blenders are not universally available in low-resourced settings and FiO2 requirement may be impacted by respiratory
support level and mean airway pressure. Importantly, there is no consensus on the optimal FiO2 at which to provide surfactant when available [9]. With the implementation of less invasive
methods of surfactant administration and ever decreasing gestational ages, a lower FiO2 cutoff is sometimes used. This controversy is evident in the RDS-NeXT guidelines where a lower FiO2
was suggested earlier (≤ 2 h of life) and a higher cutoff later (> 2 h of life) in determining the need for surfactant [26]. Despite the unclear evidence regarding the optimal FiO2
threshold at which to provide surfactant, the ability of a FiO2 threshold of 0.29 in the second hour of life to predict CPAP failure has been reported to have an AUC of 0.7 [13, 16, 28, 29].
Two other studies found that FiO2 in the first hours of life (one study combined maximum FiO2 with chest radiograph showing severe RDS) was able to predict CPAP failure with an AUC of
greater than 0.8 [10, 11]. Though not directly comparable, the simplified Downes score obtained on admission was able to predict the receipt of surfactant (after adjusting for gestational
age) with an AUC of 0.83. The MAP: FiO2 product, referred to as the respiratory severity score (RSS) has also been evaluated for its ability to predict CPAP failure and the receipt of
surfactant, with RSS cutoffs of greater than 1.28 and greater than 1.5 having been reported [12, 17, 30]. However, the optimal RSS cutoff to predict the receipt of surfactant is yet to be
determined. In our study, the mean RSS was 1.59 for infants that did not receive surfactant and 2.33 for infants that did receive surfactant, with both groups having mean RSS scores greater
than the cutoffs previously suggested in the literature [12]. In addition to needing further validation and exploration, the broad use of this score is limited due to lack of standardization
in CPAP modalities as pressure delivered varies between devices and interfaces. The de-emphasis of blood gases and chest radiographs in favor of preemptive surfactant therapy based on work
of breathing and FiO2 requirement, as mentioned in the most recent European Consensus Guidelines on the management of RDS, suggest the need for a validated clinical examination tool, such as
the Downes score [9]. The Silverman-Andersen score is another clinical examination scoring tool that has been shown to predict the need for additional respiratory support in highly
resourced settings [31, 32]. It is also being used to help identify CPAP failure and guide surfactant administration in low-resourced settings [6]. Setty and colleagues further simplified
the Silverman-Andersen score with the goal of it being easier to implement in low-resource settings [27]. However, when compared to the Silverman-Andersen score, the modified Downes score
has been suggested to be more reliable and easier to obtain [33]. In addition, the modified Downes score has been shown to have a high inter-rater reliability [22]. Our study’s limitations
lie in the fact that the Downes score was calculated and simplified from nursing admission data at a single center in a highly resourced setting. Further prospective studies should be
performed using the Downes score, or a simplified version as used in this study, to predict CPAP failure and to help guide surfactant administration. Also, this study only investigated the
simplified Downes score when applied at a single time point on admission. Although our study suggests utility in a simplified Downes score being completed on admission, due to the
progressive nature of RDS, further studies should evaluate the Downes score when used serially as it may improve the prediction of infants requiring escalation of respiratory support and/or
surfactant. As these infants are cared for by multi-disciplinary care teams, serial assessments could also be completed by nurses, respiratory therapists, advanced practice providers,
physicians, or other team members responsible for serial clinical assessments, and then discussed within the context of family-centered and goal-directed care. As the Downes score has been
previously used to assess the response to surfactant, there is likely utility in not only using the score serially before surfactant, but to use it after to assess response, need for repeat
dosing, and for weaning support [34]. Another major limitation to our study was the required simplification of the Downes score as our level III NICU does not currently use the Downes score,
and nursing electronic flowsheet data does not routinely discriminate between the severity of grunting, retractions, or degree of air entry. Due to the fact that the Downes score was
simplified from nursing admission data, it would need to be validated to determine if it maintains the high inter-rater reliability that the Downes score has previously demonstrated. If
found to demonstrate high inter-rater reliability, a simpler score may be more feasible for medical professionals with limited experience with neonatal RDS. Additionally, considering the
Downes score correlated well with blood gas parameters, the simplified Downes score should be further studied to see if it retains the ability to predict blood gas abnormalities. The use of
2000 grams as a surrogate marker for prematurity proved feasible in our study, with less than 3% of infants (n = 5) in the final cohort being greater than 37 weeks. However, the AUC did
improve after adjusting for gestational age. With the anticipated improved availability and affordability of surfactant in low- and middle-income countries (LMIC), it will be beneficial to
have a clinical examination scoring tool that does not rely on potentially unavailable resources, such as chest radiography and blood gases, to help guide clinical decision making regarding
surfactant therapy. Early identification of infants with RDS who are at risk for CPAP failure is critical to allow for earlier transport to a center that could provide surfactant therapy
and/or IMV. In low-resourced settings, where the Downes score (or Silverman-Andersen score) is already frequently employed, the optimal threshold will need to be determined. A score of ≥ 4
is commonly used to help guide respiratory support or initiation of CPAP. For infants admitted and stabilized on CPAP with a score less than 4, the high NPV suggests that these infants are
unlikely to require surfactant therapy. Although many centers in LMIC currently use the Downes score, immediate generalizability for the ability of the score to predict surfactant is
difficult due to the lower rates of antenatal corticosteroids in these settings compared to that in our study and other highly resourced settings. Per the original description of the score
by Dr Downes, “To be useful to the community pediatrician who lacks facilities for arterial pH, [arterial] pCO2 and [arterial] pO2 measurements, a clinical scoring system must (1) be simple
and easily learned; (2) involve no biochemical determinations; (3) provide some guide to treatment as well as prognosis; and (4) be related within reasonable limits to the physiologic
derangements in the infant” [18]. Even 50 years later, the Downes score and its modifications continue to be used by practitioners around the world, and now may have found new utility in
guiding surfactant administration. CONCLUSION The simplified Downes score can help predict the receipt of surfactant. The results of this study suggest that the incorporation of a reliable
clinical assessment tool, such as the original or simplified Downes score, into normal admission workflows of infants with RDS may help guide clinical decision making regarding surfactant
administration. Further studies should be performed prospectively to see if a standardized clinical assessment improves the early identification of infants at risk of requiring invasive
mechanical ventilation, and to determine who may benefit from surfactant administration. REFERENCES * Ekhaguere OA, Okonkwo IR, Batra M, Hedstrom AB. Respiratory distress syndrome management
in resource limited settings—Current evidence and opportunities in 2022. Front Pediatr. 2022;10:961509. Article PubMed PubMed Central Google Scholar * Tochie JN, Sibetcheu AT,
Arrey-Ebot PE, Choukem SP. Global, regional and national trends in the burden of neonatal respiratory failure and essentials of its diagnosis and management from 1992 to 2022: a scoping
review. Eur J Pediatr [Internet]. 2023. [cited 2023 Dec 14]. Available from: https://link.springer.com/10.1007/s00431-023-05238-z * Kamath BD, MacGuire ER, McClure EM, Goldenberg RL, Jobe
AH. Neonatal mortality from respiratory distress syndrome: lessons for low-resource countries. Pediatrics. 2011;127:1139–46. Article PubMed PubMed Central Google Scholar * Thukral A,
Sankar MJ, Chandrasekaran A, Agarwal R, Paul VK. Efficacy and safety of CPAP in low- and middle-income countries. J Perinatol. 2016;36:S21–8. Article PubMed PubMed Central Google Scholar
* Dargaville PA, Gerber A, Johansson S, De Paoli AG, Kamlin COF, Orsini F, et al. Incidence and Outcome of CPAP Failure in Preterm Infants. Pediatrics. 2016;138:e20153985. Article PubMed
Google Scholar * Abdallah Y, Mkony M, Noorani M, Moshiro R, Bakari M, Manji K. CPAP failure in the management of preterm neonates with respiratory distress syndrome where surfactant is
scarce. A prospective observational study. BMC Pediatr. 2023;23:211. Article CAS PubMed PubMed Central Google Scholar * COMMITTEE ON FETUS AND NEWBORN, Papile LA, Baley JE, Benitz W,
Cummings J, Eichenwald E, et al. Respiratory support in preterm infants at birth. Pediatrics. 2014;133:171–4. Article Google Scholar * Ng EH, Shah V. Guidelines for surfactant replacement
therapy in neonates. Paediatr Child Health. 2021;26:35–41. Article PubMed PubMed Central Google Scholar * Sweet DG, Carnielli VP, Greisen G, Hallman M, Klebermass-Schrehof K, Ozek E, et
al. European consensus guidelines on the management of respiratory distress syndrome: 2022 update. Neonatology. 2023;120:3–23. Article PubMed Google Scholar * Dargaville PA, Aiyappan A,
De Paoli AG, Dalton RGB, Kuschel CA, Kamlin CO, et al. Continuous positive airway pressure failure in preterm infants: incidence, predictors and consequences. Neonatology. 2013;104:8–14.
Article CAS PubMed Google Scholar * Kakkilaya V, Wagner S, Mangona KLM, Steven Brown L, Jubran I, He H, et al. Early predictors of continuous positive airway pressure failure in preterm
neonates. J Perinatol. 2019;39:1081–8. Article PubMed Google Scholar * Zapata H, Becker H, Lasarev M, Fort P, Guthrie S, Kaluarachchi D. Respiratory severity score during the first three
hours of life as a predictor for CPAP failure and need for late surfactant administration [Internet]. In Review; 2022. [cited 2023 Dec 14]. Available from:
https://www.researchsquare.com/article/rs-2093192/v1 * Ramaswamy VV, Bandyopadhyay T, Abiramalatha T, Pullattayil S AK, Szczapa T, Wright CJ, et al. Clinical decision thresholds for
surfactant administration in preterm infants: a systematic review and network meta-analysis. EClinicalMedicine. 2023;62:102097. Article PubMed PubMed Central Google Scholar * Guellec I,
Debillon T, Flamant C, Jarreau PH, Serraz B, Tourneux P. Management of respiratory distress in moderate and late preterm infants: clinical trajectories in the Neobs study. Eur J Pediatr.
2023;182:5661–72. Article PubMed PubMed Central Google Scholar * Dargaville PA, Kamlin COF, Orsini F, Wang X, De Paoli AG, Kanmaz Kutman HG, et al. Effect of minimally invasive
surfactant therapy vs sham treatment on death or bronchopulmonary dysplasia in preterm infants with respiratory distress syndrome: the OPTIMIST-A randomized clinical trial. JAMA.
2021;326:2478–87. Article CAS PubMed Google Scholar * Branagan A, Yu I, Gurusamy K, Miletin J. Thresholds for surfactant use in preterm neonates: a network meta-analysis. Arch Dis Child
Fetal Neonatal Ed. 2023;108:333–41. Article PubMed Google Scholar * Pillai MS, Sankar MJ, Mani K, Agarwal R, Paul VK, Deorari AK. Clinical prediction score for nasal CPAP failure in
pre-term VLBW neonates with early onset respiratory distress. J Trop Pediatr. 2011;57:274–9. Article PubMed Google Scholar * Downes JJ, Vidyasagar D, Boggs TR, Morrow GM. Respiratory
distress syndrome of newborn infants. I. New clinical scoring system (RDS score) with acid-base and blood-gas correlations. Clin Pediatr (Philos). 1970;9:325–31. Article CAS Google Scholar
* Rusmawati A, Haksari EL, Naning R. Downes score as a clinical assessment for hypoxemia in neonates with respiratory distress. Paediatr Indones. 2016;48:342. Article Google Scholar *
Shrestha S, Shrestha SK, Shrestha GS, Dhakal MS. assessment of hypoxemia using Downes score in neonates with respiratory distress. Nepal Med Coll J. 2021;23:194–7. Article Google Scholar *
John B, Venkateshwar V, Dagar V. Predictors of outcome in neonates with respiratory distress. J Nepal Paediatr Soc. 2015;35:31–7. Article Google Scholar * Ehret DEY, Demtse Gebremedhin A,
Hadgu Berhe A, Hailu Y, Metaferia G, Kessler K, et al. High inter‐rater reliability between physicians and nurses utilising modified Downes’ scores in preterm respiratory distress. Acta
Paediatr. 2023;112:2329–37. Article PubMed Google Scholar * Yuniati T, Permatagalih V, Suryaningrat FR, Wahyudi K, Kadi FA, Primadi A, et al. Downes score as a predictor of nasal
continuous positive airway pressure failure in neonates of 28 - 36 weeks gestation with respiratory distress: a survival analysis. Iran J Pediatr [Internet]. 2023;33. [cited 2023 Dec 14].
Available from: https://brieflands.com/articles/ijp-134539 * Permatahati WI, Setyati A, Haksari EL. Predictor factors of continuous positive airway pressure failure in preterm infants with
respiratory distress. Glob Pediatr Health. 2021;8:2333794X2110074. Google Scholar * Koti J, Murki S, Gaddam P, Reddy A, Dasaradha Rami Reddy M. Bubble CPAP for respiratory distress syndrome
in preterm infants. Indian Pediatr. 2010;47:139–43. Article PubMed Google Scholar * Bhandari V, Black R, Gandhi B, Hogue S, Kakkilaya V, Mikhael M, et al. RDS-NExT workshop: consensus
statements for the use of surfactant in preterm neonates with RDS. J Perinatol. 2023;43:982–90. Article PubMed PubMed Central Google Scholar * Setty SG, Batra M, Hedstrom AB. The
Silverman Andersen respiratory severity score can be simplified and still predicts increased neonatal respiratory support. Acta Paediatr. 2020;109:1273–5. Article PubMed Google Scholar *
Gulczyńska E, Szczapa T, Hożejowski R, Borszewska-Kornacka MK, Rutkowska M. Fraction of inspired oxygen as a predictor of CPAP failure in preterm infants with respiratory distress syndrome:
a prospective multicenter study. Neonatology. 2019;116:171–8. Article PubMed Google Scholar * Glaser K, Bamat NA, Wright CJ. Can we balance early exogenous surfactant therapy and
non-invasive respiratory support to optimize outcomes in extremely preterm infants? a nuanced review of the current literature. Arch Dis Child Fetal Neonatal Ed. 2023;108:554–60. Article
PubMed Google Scholar * Tourneux P, Debillon T, Flamant C, Jarreau PH, Serraz B, Guellec I. Early factors associated with continuous positive airway pressure failure in moderate and late
preterm infants. Eur J Pediatr [Internet]. 2023. [cited 2023 Dec 14]. Available from: https://link.springer.com/10.1007/s00431-023-05090-1 * Hedstrom AB, Gove NE, Mayock DE, Batra M.
Performance of the silverman Andersen respiratory severity score in predicting PCO2 and respiratory support in newborns: a prospective cohort study. J Perinatol. 2018;38:505–11. Article
PubMed PubMed Central Google Scholar * Hedstrom AB, Faino AV, Batra M. The Silverman Andersen respiratory severity score in the delivery room predicts subsequent intubation in very
preterm neonates. Acta Paediatr. 2021;110:1450–1. Article PubMed Google Scholar * Shashidhar A, Suman Rao P, Joe J. Downes Score vs Silverman Anderson score for assessment of respiratory
distress in preterm newborns. Pediatr Oncall [Internet]. 2016;13. [cited 2023 Dec 14]. Available from:
https://www.pediatriconcall.com/pediatric-journal/view/fulltext-articles/1027/J/0/0/542/0 * Macooie AA, Fakour Z, Roanaghi P. Comparative evaluation of the effects of BLES and Survanta on
treatment of respiratory distress syndrome in newborns. J Fam Med Prim Care. 2018;7:1063–7. Article Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
Department of Pediatrics, University of Vermont Larner College of Medicine, Burlington, VT, USA William Chotas, Erika M. Edwards, Delia Horn, Roger Soll & Danielle E. Y. Ehret * Vermont
Oxford Network, Burlington, VT, USA Erika M. Edwards, Roger Soll & Danielle E. Y. Ehret * University of Vermont College of Engineering and Mathematical Sciences, Burlington, VT, USA
Erika M. Edwards Authors * William Chotas View author publications You can also search for this author inPubMed Google Scholar * Erika M. Edwards View author publications You can also search
for this author inPubMed Google Scholar * Delia Horn View author publications You can also search for this author inPubMed Google Scholar * Roger Soll View author publications You can also
search for this author inPubMed Google Scholar * Danielle E. Y. Ehret View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS WC had primary
responsibility for protocol development, chart review, and writing the manuscript. DE, DH, EME, and RS participated in the development of the protocol, supervised the design and execution of
the study, and contributed to the writing of the manuscript. EME also performed the data analyses. CORRESPONDING AUTHOR Correspondence to William Chotas. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ETHICS APPROVAL The University of Vermont Institutional Review Board determined that this study was not human subjects research. This
analysis of existing data was performed in accordance with the Declaration of Helsinki. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0
International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s)
and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material
derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions
ABOUT THIS ARTICLE CITE THIS ARTICLE Chotas, W., Edwards, E.M., Horn, D. _et al._ Using a simplified Downes score to predict the receipt of surfactant in a highly resourced setting. _J
Perinatol_ 45, 30–35 (2025). https://doi.org/10.1038/s41372-024-02086-z Download citation * Received: 14 May 2024 * Revised: 25 July 2024 * Accepted: 29 July 2024 * Published: 05 August 2024
* Issue Date: January 2025 * DOI: https://doi.org/10.1038/s41372-024-02086-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable
link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative