Play all audios:
ABSTRACT Bipolar disorder (BD) is a mental disorder characterized by recurrent relapses of affective episodes, cognitive impairment, illness progression, and reduced life expectancy.
Increased systemic oxidatively generated nucleoside damage have been found in some neurodegenerative disorders and in BD. As the first, this naturalistic prospective, longitudinal follow-up
case-control study investigated cerebrospinal fluid (CSF) oxidative stress markers 8-oxo-7,8-dihydroguanosine (8-oxoGuo) and 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) that relate to RNA
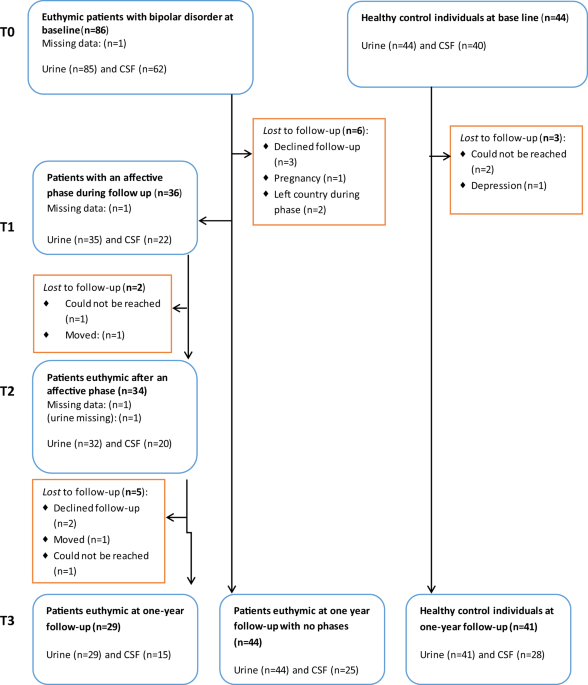
and DNA damage, respectively. Patients with BD (_n_ = 86, 51% female) and gender-and-age-matched healthy control individuals (HC; _n_ = 44, 44% female) were evaluated at baseline (T0),
during (T1) and after a new affective episode (T2), if it occurred, and after a year (T3). Cerebrospinal and urine oxidative stress markers were analyzed using ultra-performance liquid
chromatography–tandem mass spectrometry. CSF-8-oxoGuo was statistically significantly higher by 18% (_p_ = 0.003) in BD versus HC at T0, and by 22% (_p_ _=_ 0) at T3. CSF-8-oxoGuo had
increased by 15% (_p_ = 0.042) from T0 to T3, and by 14% (_p_ = 0.021) from T2 to T3 in patients, who experienced an episode during follow-up. CSF-8-oxodG had increased by 26% (_p_ = 0.054)
from T0 to T2 and decreased by 19% (_p_ = 0.041) from T2 to T3 in patients, who experienced an episode during follow-up. CSF-8-oxoGuo did not show a statistically significant change in HC
during the one-year follow-up. CSF and urine-8-oxoGuo levels correlated moderately. In conclusion, CSF oxidative stress marker of RNA damage 8-oxoGuo showed both state and trait dependence
in BD and stability in HC. Central RNA damage may be a potential biomarker for BD. SIMILAR CONTENT BEING VIEWED BY OTHERS BIOENERGETIC BIOMARKERS AS PREDICTIVE INDICATORS AND THEIR
RELATIONSHIP WITH COGNITIVE FUNCTION IN NEWLY DIAGNOSED, DRUG-NAÏVE PATIENTS WITH BIPOLAR DISORDER Article Open access 14 April 2025 BIOMARKERS IN THE CEREBROSPINAL FLUID OF PATIENTS WITH
PSYCHOTIC DISORDERS COMPARED TO HEALTHY CONTROLS: A SYSTEMATIC REVIEW AND META-ANALYSIS Article 11 May 2023 ALTERED LEVELS OF INTERLEUKINS AND NEUROTROPHIC GROWTH FACTORS IN MOOD DISORDERS
AND SUICIDALITY: AN ANALYSIS FROM PERIPHERY TO CENTRAL NERVOUS SYSTEM Article Open access 02 June 2021 INTRODUCTION Bipolar disorder (BD) is a disabling mental illness with a prevalence of
1%, a high risk of recurrence of manic and depressive episodes, a lifelong elevated risk of suicide1 and a heritability of 60–80%2. A vast body of literature evidence show clinical
progression in BD with increasing risk of developing new mood episodes with every episode, progressive shortening of inter-episode intervals with each recurrence, and with increasing
cognitive disabilities during the course of illness1,3,4,5,6,7,8. However, systematic research of the underlying neurobiology of illness progression is lacking. Elevated levels of peripheral
markers of oxidative stress have been found in psychiatric disorders, diabetes, and neurodegenerative disorders9,10,11. Oxidative stress reflects an increase in pro-oxidants, which
subsequently leads to oxidative modifications of cellular components, such as RNA and DNA12. Oxidative stress markers 8-oxo-7,8-dihydroguanosine (8-oxoGuo), a marker of RNA oxidation, and
8-oxo-7,8dihydro-2′-deoxyguanosine (8-oxodG), a marker of DNA oxidation, can reliably be quantified in cerebrospinal fluid (CSF)13 and urine14 using a modified ultra-performance liquid
chromatography and mass spectrometry assay, and are valid markers of central/whole-body RNA and DNA damage, respectively14. We and other groups have found elevated levels of urine-8-oxoGuo
and 8-oxodG in patients with BD compared to healthy control individuals (HC)9,15,16,17. Furthermore, in a longitudinal study our group has found increased oxidative stress in manic/hypomanic
states versus remission17. Postmortem measurements indicate DNA as the main site of oxidative stress modifications in the central nervous system in severe mental illnesses and suggested
that 8-oxoGuo may pass the blood–brain barrier more readily than 8-oxodG18. Data are largely missing on oxidative stress evolution during progression of BD11 and as recently reviewed, CSF
oxidative stress has not yet been investigated in either BD or HC 19. This study aimed, as the first, to investigate state-specific, intra-individual changes in repeated measures of
cerebrospinal and urinary markers of oxidative stress in outpatients diagnosed with BD compared to HC individuals during a one-year prospective, longitudinal follow-up study. The following
hypotheses were tested: Cerebrospinal and urinary oxidative stress marker levels are: (1) higher in patients with BD compared to HC, (2) stable during a year in HC, (3) increased during and
following an affective episode, and (4) correlated. PARTICIPANTS AND METHODS SETTING The study was conducted at the Copenhagen Affective Disorder Research Center. Participants for the study
were investigated from 1 April 2014 until 27 April 2017. All participants were assessed at baseline (T0) and after a follow-up of one year (T3). The mood states of patients with BD were
evaluated by weekly contacts. In case of a new affective episode of depression, hypomania or mania patients were reassessed during the episode (T1) and at the time they had regained
remission (T2; Table 1). All participants provided written informed consent and were reimbursed regarding lumbar puncture. PARTICIPANTS PATIENTS WITH BD Newly diagnosed patients aged 18–60
years with BD in remission were recruited from the Copenhagen Affective Disorder Clinic that receives patients from the Capital Region of Denmark covering 1.6 million people and all
psychiatric centres in the region20. Diagnoses were initially provided by experienced psychiatrists in the Clinic. Exclusion criteria were significant physical illness, pregnancy or planned
pregnancy within a year, substance abuse, expected noncompliance with the protocol, no informed consent, and finally practical reasons. HEALTHY CONTROL INDIVIDUALS Age-and-gender-matched HC
with no personal or first-degree family history of psychiatric disorders were recruited among blood donors aged 18–60 years affiliated to the Blood Bank at Frederiksberg Hospital, Copenhagen
as in prior studies from our group21. Exclusion criteria were the same as for the patients. CLINICAL ASSESSMENT BASELINE T0 Written and oral information of the study was given to patients
with BD at the Copenhagen Affective Disorder Clinic and at the Blood Bank for the HC followed up by a personal contact by e-mail or telephone. After giving informed consent, the participants
were examined at baseline (T0). The clinical diagnosis was evaluated using the semistructured Schedules for Clinical Assessment in Neuropsychiatry (SCAN) interview22 conducted by specialist
in psychiatry (U.K.). The severity of mood symptoms was assessed using the 17-item Hamilton Depression Rating Scale (HAMD)23 and the Young Mania Rating Scale (YMRS)24. Remission was defined
as scores below 8 on both scales for at least two weeks. Furthermore, clinical characteristics were assessed, including weight, height, current medication, alcohol consumption, smoking
habits, duration of illness from first hypomanic episode, and history of psychoses. Severity of illness was estimated using the Global Clinical Impression Scale25. FOLLOW-UP T1, T2, AND T3
All participants were followed prospectively for a year. The patients received treatment as usual and were instructed to daily self-monitoring of mood, sleep, alcohol, and medicine intake.
Psychiatrist U.K. kept in weekly contact with the patients by their choices of either telephone, short message service, or e-mail. Patients who experienced a moderate to severe affective
episode defined as scores above 13 points on either the HAMD or the YMRS for at least two weeks, had a repeated clinical assessment, including urine, blood, and CSF sampling during the
episode (T1) and, also following the episode when being in stable remission for at least two weeks (T2). Finally, all participants were assessed at the one-year follow-up in remission,
defined as at least eight weeks in a stable remission state (T3), see Flowchart, Fig. 1. On the basis of prior data from the Copenhagen Affective Disorder Clinic26, we expected that 50% of
the patients would experience an affective episode during the follow-up period. BIOLOGICAL ASSESSMENTS The participants (fasted overnight before the collection of CSF, blood, and urine
samples between 0800 and 1000 h in the morning. At all timepoints (T0, T1, T2, and T3), the clinical assessments and the urine, blood, and CSF sampling from the participants were done on the
same or the following day. SAMPLING AND HANDLING OF CSF Specialists of neurology (S.G.H. and P.R.) performed lumbar puncture to collect CSF samples from patients with BD and HC individuals
in the lateral decubitus position. The spinal needle was inserted into the L3/L4 or L4/L5 interspace, and a total volume of 10–12 ml of CSF was collected in polypropylene tubes, and gently
inverted to avoid gradient effects. Samples where centrifuged on acquisition at 2,000 _g_ for 10 min at +4 °C and stored in polypropylene tubes in 250 µL aliquots at −80 °C pending analysis.
A general CSF screen was conducted, including albumin, immunoglobulin G (IgG), IgG index, erythrocytes, white blood cells, glucose, and protein. BLOOD SAMPLING Board-certified laboratory
technicians collected blood samples that were analyzed at the Clinical Biochemical Laboratory at Rigshospitalet, Denmark, regarding standard biochemical parameters, including hematological
parameters, blood glucose, C-reactive protein, thyroid hormones, lipid status, ions, metabolites, liver enzymes, and lithium levels. URINE SAMPLING A freshly voided spot urine was obtained
using a standard sampling kit without any additives. The sample was kept on ice and centrifuged at 4 °C and 1590 _g_ for 15 min, after which aliquots of 1.5 ml were transferred to Eppendorf
tubes and stored at −80 °C pending analyses. The results for oxidative stress markers in urine were normalized for creatine27. ANALYSES OF 8-OXOGUO AND 8-OXODG The cerebrospinal and urinary
oxidative stress markers 8-oxoGuo and 8-oxodG were analyzed at Laboratory of Clinical Pharmacology, Rigshospitalet using ultra-performance liquid chromatography–tandem mass spectrometry, as
described in full detail elsewhere 13,27. STATISTICAL ANALYSES Data were analyzed according to a preestablished protocol. All analyses were conducted with SAS software, version 9.4,
(Copyright 2013, SAS Institute Inc., Cary, NC, USA). All _p_ values were corrected for multiple testing using the Benjamini & Hochberg procedure28. We applied a conservative cutoff for
the false discovery rate at 0.05, which limits the rate of false positives among the reported findings to one in 20, so that an adjusted _p_ ≤ 0.05 was considered statistically significant.
All biomarkers were found to have a skew distribution and were therefore log-transformed prior to analysis. Hence, estimated differences between groups and timepoints are expressed in
relative terms as percent-wise differences. Regarding sample size and power, the numbers of participants in this present study, including 86 patients with BD and 44 HC individuals are like
the largest prior case-control studies, regarding peripheral oxidative stress markers in BD15,16,17. DEMOGRAPHIC AND CLINICAL DATA Demographic and clinical data at timepoints T0, T2, and T3
were summarized in numbers and percentages (categorical data), means and s.d. (normally distributed continuous data), and medians and quartiles (non-normally distributed continuous data).
Comparisons of BD and HC at T0 and T3 was made using Fisher’s exact test, Welch’ _t_-test or the Mann–Whitney _U_ test, whichever was most appropriate. MARKERS OF OXIDATIVE STRESS IN BD AND
HC AT BASELINE AND AT THE ONE-YEAR FOLLOW-UP To compare biomarker levels of CSF-8-oxoGuo, CSF-8-oxodG, urine-8-oxoGuo, and urine-8-oxodG between BD and HC, a linear mixed model was applied
with time (T0 or T3) and group (BD or HC) as fixed effects and with an unstructured covariance to account for correlation between the repeated measurements on the study participants. The
analyses were performed in three versions, version 1: no adjustment for potential confounders; version 2: adjusted for gender, age, and body mass index (BMI); version 3: adjusted for gender,
age, BMI, alcohol consumption, and smoking. Estimated differences between BD and HC are reported for biomarker levels at T0, biomarker levels at T3, and change in biomarker level from T0 to
T3. The analyses were repeated with further stratification of BD into the participants who either had or had not experienced an episode during follow-up. Internal validity of the measured
biomarkers was evaluated by comparing their levels at baseline and follow-up in HC. PATIENTS WITH BD, WHO HAD AN AFFECTIVE EPISODE DURING FOLLOW-UP A subgroup analysis was performed to
evaluate changes in biomarker levels in patients with BD, who had experienced an affective episode during follow-up. To this end, a linear mixed model with timepoint (T0, T1, T2, T3) as
fixed effect and an unstructured covariance was applied. Estimates were reported for changes between the timepoints. The analysis was performed in two versions, version 1: no adjustment for
potential confounders; and version 2: adjusted for gender, age, BMI, and the three mood stabilizers lithium, quetiapine, and lamotrigine. CORRELATIONS BETWEEN CSF AND URINARY MEASURES OF
OXIDATIVE STRESS Spearman and Pearson correlations were estimated between CSF and urine 8-oxoGuo and 8-oxodG at timepoint T0 and T3, and in BP and HC separately. EFFECT OF DOSE OF LITHIUM,
QUETIAPINE, LAMOTRIGINE AND SMOKING ON CSF AND URINARY MEASURES OF OXIDATIVE STRESS To investigate the effect of the three mood stabilizers, we applied a linear mixed model as previously
described29, which distinguishes the cross-sectional effect (i.e., the effect of the average dose of the drug over time) from the longitudinal effect (i.e., the effect of changes in the dose
of the drug over time) in order to address potential biases due to unmeasured confounders. In mixed models, the effect of smoking on each of the four outcomes was estimated with inclusion
of data from all four timepoints. SENSITIVITY ANALYSES All analyses were repeated including and excluding outliers. This did not alter the results to any significant extent. We report data
including outliers. RESULTS INCLUSION, DEMOGRAPHICS, CLINICAL, AND STUDY CHARACTERISTICS Out of a total of 497 eligible patients with BD, 86 patients were included in the study in remission.
A total of 411 did not enter the study due to: not obtaining remission before the inclusion ended in January 2016 (_n_ = 57), significant physical illness (_n_ = 97), pregnancy or planned
pregnancy (_n_ = 62), substance abuse (_n_ = 26), expected noncompliance with the protocol (_n_ = 90), not giving informed consent (_n_ = 49), and discharge from the clinic before an
informed consent could be obtained (_n_ = 30). Demographics and clinical characteristics of the participants of the study are presented in Table 1. A total of 24 participants (BD = 15, HC =
9) received medical treatment for a stabilized physical disorder or as hormone anticonception: hypertension (BD = 1), diabetes mellitus type II (BD = 1), hypothyroidism (BD = 3, HC = 1), and
hormonal contraceptives (BD = 10, HC = 9). Patients were most frequently treated with lithium, lamotrigine, and quetiapine, but three patients did not get any psychotropic medication at
inclusion. A total of 44 HC were included in the study. Patients with BD and HC individuals were well matched according to age, gender and, BMI at baseline and there were no differences
either at follow-up. There were more smokers among patients with BD at baseline and at follow-up alcohol intake was higher in HC individuals. The flow chart (Fig. 1) shows that 36 patients
with BD developed a new affective episode during follow-up and of these 34 reached stable remission within the study period. The completion rates from baseline to follow-up for patients with
BD and HC were 65% versus 86% regarding CSF, and 70% versus 93% regarding urine samples. All together 62 patients with BD and 40 HC gave samples of both CSF and urine at baseline. All
participants, but one, provided a urine sample at baseline (BD = 85, HC = 44). LEVELS OF CEREBROSPINAL AND URINARY OXIDATIVE STRESS MARKER LEVELS IN PATIENTS WITH BD COMPARED TO HC
CSF-8-oxoGuo was statistically significantly higher by 18% (95% confidence interval (CI) 8–28%, adj-_p_ = 0.003) in patients with BD versus HC at baseline, and by 22% (95% CI 12–34%, adj-_p_
= 0) at follow-up. Urine-8-oxoGuo was statistically significantly higher by 17% (95% CI 8–27%, adj-_p_ = 0.003) in patients with BD versus HC at baseline, and by 30% (95% CI 16–45%, adj-_p_
= 0) at follow-up. CSF-8-oxodG was statistically significantly higher by 29% (95% CI 6–55%, adj-_p_ = 0.043) at baseline, and urine-8-oxodG was statistically significantly higher by 25%
(95% CI 10–43%, adj-_p_ = 0.005) at follow-up in BD versus HC. CSF-8-oxodG was higher by 13% at follow-up and urine-8-oxodG was higher by 12% at baseline in patients with BD versus HC, but
these differences were not statistically significant in the adjusted models (Fig. 2 and Table 2). When patients with BD were divided into subgroups with and without a new affective episode
during follow-up, no statistically significant differences in the oxidative stress marker levels were found between the subgroups at the timepoints baseline (T0) and follow-up (T3)
(Supplemental Table 1). Furthermore, when considering the relative changes from T0 to T3 in the subgroups with and without an episode during follow-up compared to HC, no differences were
found in the relative changes between T0 and T3 (Supplemental Table 2). Regarding the internal validity of the biomarkers only CSF-8-oxoGuo did not show a statistically significant change in
HC during the one-year follow-up (Supplemental Table 3). On the contrary, CSF-8-oxodG in HC increased significantly by 22% while urine-8-oxoGuo and urine-8-oxodG decreased significantly by
15 and 14%, respectively (Supplemental Table 3). CHANGES IN LEVELS OF CEREBROSPINAL AND URINARY OXIDATIVE STRESS MARKER LEVELS IN PATIENTS WITH BD DURING AND FOLLOWING AN AFFECTIVE EPISODE
CSF-8-oxoGuo had increased by 15% (95% CI 4–27%, adj-_p_ = 0.042) from T0 to T3, and by 14% (95% CI 6–23%, adj-_p_ = 0.021) from T1 (during an episode) to T3 in patients who experienced an
episode during follow-up (Table 3). CSF-8-oxodG had increased by 26% (95% CI 5–51%, adj-_p_ = 0.054) from T0 to T2 (after an episode) and decreased by 19% (95% CI −30–6%, adj-_p_ = 0.041)
from T2 to T3 in patients who experienced an episode during follow-up. No statistically significant changes were found in the other CSF and urinary oxidative stress markers or between any of
the remaining timepoints (Table 3). CORRELATIONS BETWEEN CEREBROSPINAL AND URINARY OXIDATIVE STRESS MARKERS Measures of cerebrospinal and urinary oxidative stress markers of nucleoside
damage correlated in separate analyses of all participants, patients with BD and HC (Supplemental Table 4). Strong statistically significant correlations were found between CSF-8-oxoGuo and
CSF-8-oxodG in patients, with BD and HC individuals at both baseline and follow-up. Weak to moderate statistically significant correlations were found between CSF-8-oxoGuo and urine-8-oxoGuo
in patients, with BD and HC individuals at both baseline and follow-up. Furthermore, moderate statistically significant correlations were found between CSF-8-oxodG and urine-8-oxodG in
patients, with BD and HC individuals at baseline and follow-up regarding HC individuals. However, a weak correlation between CSF-8-oxodG and urine-8-oxodG was not statistically significant
in patients with BD at follow-up (Supplemental Table 4). THE INFLUENCE OF MEDICATION ON OXIDATIVE STRESS MARKERS Measures of cerebrospinal and urinary oxidative stress markers of nucleoside
damage tended to increase with increasing doses of lithium. The longitudinal effect, i.e., the effect of individual changes in dose of lithium was the strongest on CSF-8-oxoGuo (+0.9% per
mmol increase in dose, 95% CI 0.3–1.6%, _p_-adj = 0.04) and remained statistically significant in multivariate analysis and after adjustment for multiple testing. A similar effect was only
borderline statistically significant on CSF-8-oxodG (+1.2% per mmol increase in dose, 95% CI 0.3–2.2%, _p_-adj = 0.06). No significant effects of increasing doses of quetiapine and
lamotrigine were found in 8oxoGuo and 8oxodG in either CSF or urine (Supplemental Table 5). THE INFLUENCE OF OTHER COVARIATES ON OXIDATIVE STRESS MARKERS IN PATIENTS WITH BD As predicted,
measures of cerebrospinal and urinary oxidative stress markers of nucleoside damage depend on age and gender. However, there was no general effect of other covariates, including smoking and
alcohol (Supplemental Table 6). DISCUSSION This study confirmed that levels of CSF-8-oxoGuo: (1) were statistically significantly higher at both baseline and follow-up in patients with BD
compared to HC, (2) showed internal validity since the values in HC did not change from baseline to follow-up, (3) increased following an affective episode in patients with BD, and (4)
correlated moderately with levels of urine-8-oxoGuo. Thus, cerebrospinal oxidative stress markers of RNA damage 8-oxoGuo showed both state and trait dependence in BD and stability in HC. In
subgroup comparisons between patients with BD either with or without an episode during follow-up, a new affective episode was not predictable from baseline levels of oxidative stress
markers. Explorative analyses showed that increasing doses of lithium were associated with an increase of cerebrospinal and urinary oxidative stress markers, but this may likely be a result
of confounding by indication (higher doses of lithium prescribed for more severe BDs). Furthermore, prior studies suggested that lithium was associated with decreased peripheral oxidative
stress marker levels in euthymic patients with BD30,31,32. This study found different pathophysiological characteristics in centrally and systemically generated oxidative stress. The results
suggest that a possible pathogenic effect may be found down-stream from DNA, since no statistically significant differences between BD and HC were found regarding DNA damage measured by
8-oxodG, but merely in 8-oxoGuo that represents RNA damage. Also, the only weak to moderate correlation between centrally and systemically generated oxidative stress emphasize a role of the
blood–brain barrier. The findings regarding urinary oxidative stress from the present study are consistent with prior findings from our group, showing increased levels of urine markers of
oxidative stress in unipolar depression33, BD rapid cycling16, BD type I17, and schizophrenia34. In these prior studies, state dependencies were found only in patients diagnosed with BD type
I between the states of mania and stable remission17. Increased levels of oxidative stress may predict mortality in patients with other chronic diseases such as diabetes35. Epidemiological
studies have found that the life expectancy among patients with BD is decreased by 8–12 years compared to the general population36 and that patients die due to natural causes of death
already from adolescence37. It is a possibility that increased levels of oxidative stress seen in patients with BD may contribute to the decreased life expectancy by inducing accelerated
ageing. The present results suggest that relapses of affective episodes may increase oxidative stress. This gives hope that effective long-term preventive treatment may contribute to
normalized life expectancy in BD. Furthermore, our findings suggest that CSF oxidative stress may represent state (increased at T1) and trait markers (increased at T2 and T3) in BD, and may
reflect neurobiological correlates of illness progression and sensitization5,38 in BD. Mitochondrial dysfunction reflected as increased oxidative stress may be a biological underpinning of
BD11,39, and impaired autophagy has been suggested as the link between mitochondrial dysfunction and psychiatric disorders11. Overall, our findings show that central RNA damage may be
related to the pathogenesis of BD. LIMITATIONS Only a total of 36 patients (42%) experienced an affective episode during the follow-up period and the subjects had fewer repeated CSF samples
than urine samples. Data were analyzed using linear mixed models, which implicitly imputes missing data from missed samples and drop outs, and provides unbiased results under the assumption
that missing data is missing at random. However, results may still be biased if missingness depends on confounding factors that are not accounted for. Smoking was more prevalent in BD
compared to HC. However, analyses of the effect of smoking showed no significant effect in uni- and mulitivariate analyses. Estimates and _p_ values remained significant after adjusting for
alcohol and smoking. Clinical researchers (U.K., S.G.H., and P.R.) were not blinded to the participant being BD or HC. However, they did not participate in the statistical analyses of the
oxidative stress markers that for all practical reasons were blinded. REFERENCES * Kessing, L. V., Hansen, M. G., Andersen, P. K. & Angst, J. The predictive effect of episodes on the
risk of recurrence in depressive and bipolar disorders - a life-long perspective. _Acta Psychiatr. Scand._ 109, 339–344 (2004). Article CAS Google Scholar * Lohoff F. W. B. W. _Genetics
of Bipolar disorder: Clinical and Neurobiological Foundations_ (ed Yatham, L. N. M. M.) (Wiley-Blackwell, Singapore, 2010). * Kessing, L. V., Andersen, P. K., Mortensen, P. B. & Bolwig,
T. G. Recurrence in affective disorder. I. Case register study. _Br. J. Psychiatry_ 172, 23–28 (1998). Article CAS Google Scholar * Kessing, L. V. & Andersen, P. K. Predictive effects
of previous episodes on the risk of recurrence in depressive and bipolar disorders. _Curr. Psychiatry Rep._ 7, 413–420 (2005). Article Google Scholar * Post, R. M., Fleming, J. &
Kapczinski, F. Neurobiological correlates of illness progression in the recurrent affective disorders. _J. Psychiatr. Res._ 46, 561–573 (2012). Article Google Scholar * Schneider, M. R.,
DelBello, M. P., McNamara, R. K., Strakowski, S. M. & Adler, C. M. Neuroprogression in bipolar disorder. _Bipolar Disord._ 14, 356–374 (2012). Article Google Scholar * Rizzo, L. B. et
al. The theory of bipolar disorder as an illness of accelerated aging: implications for clinical care and research. _Neurosci. Biobehav. Rev._ 42, 157–169 (2014). Article Google Scholar *
Kessing, L. V. & Andersen, P. K. Evidence for clinical progression of unipolar and bipolar disorders. _Acta Psychiatr. Scand._ 135, 51–64 (2017). Article CAS Google Scholar * Raza, M.
U., Tufan, T., Wang, Y., Hill, C. & Zhu, M. Y. DNA damage in major psychiatric diseases. _Neurotox. Res._ 30, 251–267 (2016). Article CAS Google Scholar * Cao, B. et al. Metabolic
profiling for water-soluble metabolites in patients with schizophrenia and healthy controls in a Chinese population: a case-control study. World J. Biol. Psychiatry 1–11 (2019). [Epub ahead
of publication]. * Kim, Y. et al. Mitochondria, metabolism, and redox mechanisms in psychiatric disorders. _Antioxid. Redox Signal._ 31, 275–317 (2019). Article CAS Google Scholar *
Poulsen, H. E. et al. RNA modifications by oxidation: a novel disease mechanism? _Free Radic. Biol. Med._ 52, 1353–1361 (2012). Article CAS Google Scholar * Weimann, A., Simonsen, A. H.
& Poulsen, H. E. Measurement of 8-oxo-7,8-dihydro-2’-deoxyguanosine and 8-oxo-7,8-dihydro-guanosine in cerebrospinal fluid by ultra performance liquid chromatography-tandem mass
spectrometry. _J. Chromatogr. B Anal. Technol. Biomed. Life Sci._ 1073, 110–117 (2018). Article CAS Google Scholar * Weimann, A., Belling, D. & Poulsen, H. E. Quantification of
8-oxo-guanine and guanine as the nucleobase, nucleoside and deoxynucleoside forms in human urine by high-performance liquid chromatography-electrospray tandem mass spectrometry. _Nucleic
acids Res._ 30, E7 (2002). Article Google Scholar * Brown, N. C., Andreazza, A. C. & Young, L. T. An updated meta-analysis of oxidative stress markers in bipolar disorder. _Psychiatry
Res._ 218, 61–68 (2014). Article CAS Google Scholar * Munkholm, K., Poulsen, H. E., Kessing, L. V. & Vinberg, M. Elevated levels of urinary markers of oxidatively generated DNA and
RNA damage in bipolar disorder. _Bipolar Disord._ 17, 257–268 (2015). Article CAS Google Scholar * Jacoby, A. S., Vinberg, M., Poulsen, H. E., Kessing, L. V. & Munkholm, K. Increased
DNA and RNA damage by oxidation in patients with bipolar I disorder. _Transl. Psychiatry_ 6, e867 (2016). Article CAS Google Scholar * Christensen, M. R. et al. Elevated levels of
8-oxoGuo and 8-oxodG in individuals with severe mental illness - an autopsy-based study. _Free Radic. Biol. Med._ 126, 372–378 (2018). Article CAS Google Scholar * Knorr, U. et al.
Biomarkers in cerebrospinal fluid of patients with bipol ar disorder versus healthy individuals: a systematic review. _Eur. Neuropsychopharmacol._ 28, 783–794 (2018). Article CAS Google
Scholar * Kessing, L. V. et al. The Bipolar Illness Onset study: research protocol for the BIO cohort study. _BMJ Open_ 7, e015462 (2017). Article Google Scholar * Knorr, U. et al.
Increased blood BDNF in healthy individuals with a family history of depression. _Psychiatry Res._ 256, 176–179 (2017). Article Google Scholar * Wing, J. K. et al. SCAN. Schedules for
Clinical Assessment in Neuropsychiatry. _Arch. Gen. Psychiatry_ 47, 589–593 (1990). Article CAS Google Scholar * Hamilton, M. A rating scale for depression. _J. Neurol. Neurosurg.
Psychiatry_ 23, 56–62 (1960). Article CAS Google Scholar * Young, R. C., Biggs, J. T., Ziegler, V. E. & Meyer, D. A. A rating scale for mania: reliability, validity and sensitivity.
_Br. J. Psychiatry_ 133, 429–435 (1978). Article CAS Google Scholar * Beck, A. T., Ward, C. H., Mendelson, M., Mock, J. & Erbaugh, J. An inventory for measuring depression. _Arch.
Gen. Psychiatry_ 4, 561–571 (1961). Article CAS Google Scholar * Kessing, L. V. et al. Treatment in a specialised out-patient mood disorder clinic v. standard out-patient treatment in the
early course of bipolar disorder: randomised clinical trial. _Br. J. Psychiatry_ 202, 212–219 (2013). Article Google Scholar * Rasmussen, S. T. et al. Simvastatin and oxidative stress in
humans: a randomized, double-blinded, placebo-controlled clinical trial. _Redox Biol._ 9, 32–38 (2016). Article CAS Google Scholar * Benjamini, Y., Drai, D., Elmer, G., Kafkafi, N. &
Golani, I. Controlling the false discovery rate in behavior genetics research. _Behavi. Brain Res._ 125, 279–284 (2001). Article CAS Google Scholar * Fitzmaurice, G. M., Laird, N. M.
& Ware, J. M. _Applied Longitudinal Analysis_ 2nd edn, ch. 9 (2011). * Bengesser, S. A. et al. Mood stabilizers, oxidative stress and antioxidative defense in Euthymia of bipolar
disorder. _CNS Neurol. Disord. Drug Targets_ 15, 381–389 (2016). Article CAS Google Scholar * Cui, J., Shao, L., Young, L. T. & Wang, J. F. Role of glutathione in neuroprotective
effects of mood stabilizing drugs lithium and valproate. _Neuroscience_ 144, 1447–1453 (2007). Article CAS Google Scholar * Machado-Vieira, R. et al. Oxidative stress parameters in
unmedicated and treated bipolar subjects during initial manic episode: a possible role for lithium antioxidant effects. _Neurosci. Lett._ 421, 33–36 (2007). Article CAS Google Scholar *
Jorgensen, A. et al. Systemic oxidatively generated DNA/RNA damage in clinical depression: associations to symptom severity and response to electroconvulsive therapy. _J. Affect. Disord._
149, 355–362 (2013). Article CAS Google Scholar * Jorgensen, A. et al. Increased systemic oxidatively generated DNA and RNA damage in schizophrenia. _Psychiatry Res._ 209, 417–423 (2013).
Article CAS Google Scholar * Kjaer, L. K. et al. Cardiovascular and all-cause mortality risk associated with urinary excretion of 8-oxoGuo, a biomarker for RNA oxidation, in patients
with type 2 diabetes: a prospective cohort study. _Diabetes Care_ 40, 1771–1778 (2017). Article Google Scholar * Kessing, L. V., Vradi, E. & Andersen, P. K. Life expectancy in bipolar
disorder. _Bipolar Disord._ 17, 543–548 (2015). Article Google Scholar * Kessing, L. V., Vradi, E., McIntyre, R. S. & Andersen, P. K. Causes of decreased life expectancy over the life
span in bipolar disorder. _J. Affect. Disord._ 180, 142–147 (2015). Article Google Scholar * Post, R. M. Epigenetic basis of sensitization to stress, affective episodes, and stimulants:
implications for illness progression and prevention. _Bipolar Disord._ 18, 315–324 (2016). Article CAS Google Scholar * Yoshimi, N. et al. Cerebrospinal fluid metabolomics identifies a
key role of isocitrate dehydrogenase in bipolar disorder: evidence in support of mitochondrial dysfunction hypothesis. _Mol. Psychiatry_ 21, 1504–1510 (2016). Article CAS Google Scholar
Download references ACKNOWLEDGEMENTS We thank Oda Jakobsen and Kathrine Bjarnø from the Danish Dementia Research Centre, Department of Neurology, Rigshospitalet University of Copenhagen,
Jytte Rasmussen and Pia Weikop at the Neuropsychiatric Laboratory, Rigshospitalet University of Copenhagen, Allan Hansen and Anne Præstegaard, and Agnete Mehlsen and Katja Luntang
Christensen from CADIC and Laboratory of Clinical Pharmacology for technical support. The study was supported by The Mental Health Services of Capital of Denmark Research Foundation, AP
Møller Foundation for Promotion of Medical Science, The Beckett Foundation, The King Christian 10th Foundation and the Max and Oda Wørzner Foundation (recipient author U.K.). The Danish
Dementia Research Centre is supported by grants from the Danish Ministry of Health (J No. 2007-12143-112, project 59506/J No. 0901110, project 34501) and the Danish Health Foundation (J No.
2007B004). The funding sources of the study had no role in study design; in the collection, analysis and interpretation of data; in writing of the report; and in the decision to submit the
paper for publication. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Copenhagen Affective Disorder Research Center (CADIC), Psychiatric Center Copenhagen, University of Copenhagen, Faculty
of Health and Medical Sciences, Copenhagen, Denmark Ulla Knorr, Ellen-Margrethe Christensen, Maj Vinberg, Rie Lambæk Mikkelsen, Thomas Kirkegaard, Rasmus Nejst Jensen & Lars Vedel
Kessing * Danish Dementia Research Centre, section 6922, Rigshospitalet, University of Copenhagen, Faculty of Health and Medical Sciences, Copenhagen, Denmark Anja Hviid Simonsen, Peter Roos
& Steen Gregers Hasselbalch * Laboratory of Clinical Pharmacology, Rigshospitalet, University of Copenhagen, Faculty of Health and Medical Sciences, Copenhagen, Denmark Allan Weimann
& Trine Henriksen * Department of Clinical Pharmacology, Bispebjerg Hospital, University of Copenhagen, Faculty of Health and Medical Sciences, Copenhagen, Denmark Allan Weimann, Trine
Henriksen & Henrik Enghusen Poulsen * Section of Biostatistics, Department of Public Health, University of Copenhagen, Copenhagen, Denmark Morten Akhøj & Julie Forman Authors * Ulla
Knorr View author publications You can also search for this author inPubMed Google Scholar * Anja Hviid Simonsen View author publications You can also search for this author inPubMed Google
Scholar * Peter Roos View author publications You can also search for this author inPubMed Google Scholar * Allan Weimann View author publications You can also search for this author
inPubMed Google Scholar * Trine Henriksen View author publications You can also search for this author inPubMed Google Scholar * Ellen-Margrethe Christensen View author publications You can
also search for this author inPubMed Google Scholar * Maj Vinberg View author publications You can also search for this author inPubMed Google Scholar * Rie Lambæk Mikkelsen View author
publications You can also search for this author inPubMed Google Scholar * Thomas Kirkegaard View author publications You can also search for this author inPubMed Google Scholar * Rasmus
Nejst Jensen View author publications You can also search for this author inPubMed Google Scholar * Morten Akhøj View author publications You can also search for this author inPubMed Google
Scholar * Julie Forman View author publications You can also search for this author inPubMed Google Scholar * Henrik Enghusen Poulsen View author publications You can also search for this
author inPubMed Google Scholar * Steen Gregers Hasselbalch View author publications You can also search for this author inPubMed Google Scholar * Lars Vedel Kessing View author publications
You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Ulla Knorr. ETHICS DECLARATIONS CONFLICT OF INTEREST Authors U.K., A.H.S., S.G.H., H.E.P.,
P.R., T.H., A.W., E.M.C., R.L.M., R.N.J., T.K., M.A., and J.F. reported no biomedical financial interests or potential conflicts of interest. E.M.C. reported having received a fee from
Lundbeck for teaching a group of doctors within the last year. M.V. and L.V.K. reported having been a consultant for Lundbeck within the preceding three years. ETHICAL APPROVAL The study was
approved by the Local Ethical Committee (H-6-2014-006) and the Danish Data Protection Agency, Capital Region of Copenhagen. The study complied with the latest Declaration of Helsinki.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMETATRY TABLES 1–6 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Knorr, U., Simonsen, A.H., Roos, P. _et al._ Cerebrospinal fluid oxidative stress metabolites in patients with bipolar disorder and healthy controls: a longitudinal
case-control study. _Transl Psychiatry_ 9, 325 (2019). https://doi.org/10.1038/s41398-019-0664-6 Download citation * Received: 12 September 2019 * Revised: 24 September 2019 * Accepted: 01
November 2019 * Published: 28 November 2019 * DOI: https://doi.org/10.1038/s41398-019-0664-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative