Play all audios:
ABSTRACT Renovascular hypertension (RVH) is one of the most common causes of secondary hypertension and can result in resistant hypertension. RVH is associated with an increased risk for
progressive decline in renal function, cardiac destabilization syndromes including “flash” pulmonary edema, recurrent congestive heart failure, and cerebrocardiovascular disease. The most
common cause of renal artery stenosis (RAS) is atherosclerotic lesions, followed by fibromuscular dysplasia. The endovascular technique of percutaneous transluminal renal angioplasty (PTRA)
with or without stenting is one of the standard treatments for RAS. Randomized controlled trials comparing medical therapy with PTRA to medical therapy alone have failed to show a benefit of
PTRA; however, the subjects of these randomized clinical trials were limited to atherosclerotic RAS patients, and patients with the most severe RAS, who would be more likely to benefit from
PTRA, might not have been enrolled in these trials. This review compares international guidelines related to PTRA, reevaluates the effects of PTRA treatment on blood pressure and renal and
cardiac function, discusses strategies for the management of RVH patients, and identifies factors that may predict which patients are most likely to benefit from PTRA. You have full access
to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS THE IMPACT OF RENAL ARTERY STENTING ON THERAPEUTIC AIMS Article Open access 16 December 2022
COMBINATION OF MEDICAL THERAPY AND PERCUTANEOUS TRANSLUMINAL RENAL ANGIOPLASTY VERSUS MEDICAL THERAPY ALONE FOR PATIENTS WITH ATHEROSCLEROTIC RENAL ARTERY STENOSIS: SYSTEMATIC REVIEW AND
META-ANALYSIS Article 04 March 2025 ASPECTS OF RENAL FUNCTION AND RENAL ARTERY ANATOMY AS INDICATIONS FOR RENAL DENERVATION Article 29 August 2024 INTRODUCTION Renovascular hypertension
(RVH) is one of the most common causes of secondary hypertension and can result in resistant (refractory) hypertension. RVH is associated with an increased risk of progressive decline in
renal function, cardiac destabilization syndromes including “flash” pulmonary edema, recurrent congestive heart failure (CHF), and cerebrocardiovascular disease. The most common cause of
renal artery stenosis (RAS) is atherosclerotic lesions (ARAS), followed by fibromuscular dysplasia (FMD). Although their prevalence is lower, a variety of other causes, such renal artery
dissection and embolic disease, can produce the same symptoms [1]. The endovascular technique of percutaneous transluminal renal angioplasty (PTRA) with or without stenting is one of the
standard treatments for RAS. Despite prospective clinical trial evidence for the safety and efficacy of PTRA with stenting [2,3,4], randomized controlled trials (RCTs) have shown no
difference in outcomes between medical therapy plus PTRA and medical therapy alone [5,6,7]. These negative trials led to the widespread impression that PTRA is not beneficial, and evaluation
of renal blood flow makes no sense. However, these trials had significant design flaws, and the selection of patients for PTRA is a controversial topic, as acknowledged by a comparative
effectiveness review of management strategies for RAS by the Agency for Healthcare Research and Quality [8, 9]. In this review, we compare international guidelines related to PTRA,
reevaluate the effects of PTRA treatment on blood pressure (BP) and renal and cardiac function, discuss strategies for the management of RVH patients, and identify factors that may predict
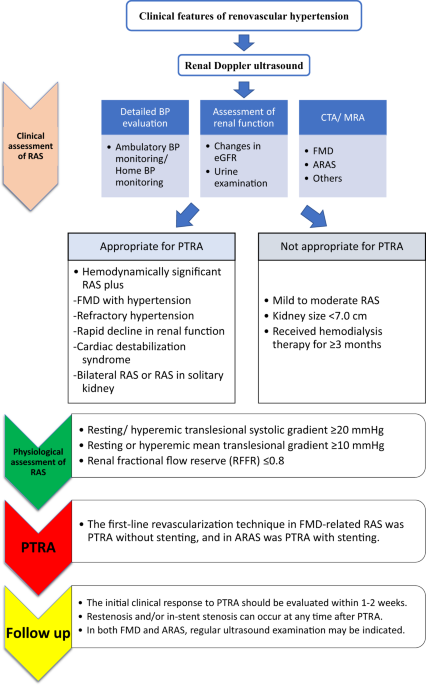
which patients are most likely to benefit from PTRA. INDICATIONS FOR REVASCULARIZATION IN THE GUIDELINES LIKELY TO BENEFIT FROM REVASCULARIZATION Most guidelines consistently recommend that
the patients most likely to benefit from revascularization have hemodynamically significant RAS and (1) FMD with hypertension, (2) resistant hypertension with failure of or intolerance to
antihypertensive medication, (3) recurrent heart failure or sudden-onset “flash” pulmonary edema, (4) progressive renal function decline due to bilateral lesions or solitary kidney, or (5)
refractory acute coronary syndrome (ACS) (Table 1) [10,11,12,13]. The recommendations from the Society for Cardiovascular Angiography and Interventions present actual figures of kidney
function (i.e., estimated glomerular filtration rate (eGFR) level or kidney size) and stenosis severity (pressure gradient). UNLIKELY TO BENEFIT FROM REVASCULARIZATION When evaluating an RAS
patient with any symptoms, it is important to diagnose whether the symptoms are caused by renal hypoperfusion. In some cases, RAS is found incidentally on routine abdominal imaging when
evaluating a patient for other problems. There is no evidence or consensus on the treatment of asymptomatic RAS patients [10]. ARAS patients with controlled BP and stable renal function on
low-dose antihypertensive drugs are unlikely to benefit from PTRA. Therefore, optimizing medical therapy should be the initial step for most RAS patients. A lack of response to medical
therapy is often an important element in deciding on revascularization. Patients with severely reduced renal function who are unlikely to benefit from PTRA include those with chronic kidney
disease (CKD) stage 5 and a pole-to-pole kidney size <7.0 cm or those receiving hemodialysis therapy for ≥3 months [10]. EFFECTS OF PTRA ON BP CONTROL AND KIDNEY AND CARDIAC FUNCTION
LIMITATIONS OF PREVIOUS RCTS Prospective RCTs reported between 1998 and 2017 failed to identify additional benefits for ARAS from endovascular stent revascularization when added to medical
therapy [5,6,7]. The subjects of these RCTs were limited to ARAS patients. The Angioplasty and Stent for Renal Artery Lesions (ASTRAL) study included 806 ARAS patients [5]. Only 60% of the
subjects in the intervention group had severity of stenosis >60%, and the rate of periprocedural complications (9%) was much higher than that in other reported studies. Patients were only
enrolled in this trial if their clinician was uncertain as to whether PTRA would be of clinical benefit, and patients who were most likely to benefit from PTRA might have been excluded from
the study. A similar limitation was found in the STent placement and BP and lipid-lowering for the prevention of progression of renal dysfunction caused by Atherosclerotic ostial stenosis
of the Renal artery (STAR) trial, which included 140 ARAS patients with creatinine clearance <80 mL/min/1.73 m2 with ostial stenosis ≥50% and controlled BP <140/90 mmHg [6]. In this
study, all patients had controlled BP, and a considerable number of participants (32% of the medication group and 34% of the stent group) had moderate (50–70%) stenosis. The Cardiovascular
Outcomes in Renal Atherosclerotic Lesions (CORAL) study enrolled 947 hypertensive patients with ARAS, and the original study protocol defined eligible candidates as those with systolic BP
≥155 mmHg despite taking ≥2 antihypertensive drugs or those with CKD defined as eGFR <60 ml/min/1.73 m2 [7]. However, because of slow enrollment, these original inclusion criteria were
later relaxed, and participants without hypertension or CKD were also recruited. Mean renal stenosis defined by core laboratory parameters in the stenting plus medical therapy group and
medical therapy group was present in 67.3% and 66.9% of subjects, respectively, which suggests that a significant proportion of ARAS patients with mild-to-moderate stenosis were included.
The number of antihypertensive medications was 2.1 ± 1.6 at baseline and increased in both the groups to 3.5 ± 1.4 in the medical therapy group and 3.3 ± 1.5 in the stenting group at the
completion of the trial. Both groups had similar decreases in systolic BP (15.6 ± 25.8 mmHg in the medical therapy group; 16.6 ± 21.2 mmHg in the stent group), implying that antihypertensive
therapy was not optimized at the initiation of treatment. The RAndomized, multi-center, prospective study comparing best medical treatment versus best medical treatment plus renal artery
stenting in patients with hemoDynamically relevant atherosclerotic renal ARtery stenosis (RADAR) study included 86 ARAS patients and aimed to evaluate the clinical impact of PTRA stenting on
eGFR in patients with hemodynamically significant ARAS based on duplex ultrasonographic patient screening [14]. However, because of slow enrollment, this study was terminated early after
the inclusion of 86 of the scheduled 300 patients (28.7%), and the outcomes of PTRA were similar to those of medical treatment. As frequently pointed out by other researchers [8,
15,16,17,18], all these recent RCTs have significant flaws, such as variability in inclusion and exclusion criteria, inconsistent definitions of improvement, or mixtures of hypertension and
renal function endpoints. Further, patients selected for RCTs represent “mild RVH” with easily achieved BP control, relatively preserved renal function, and absence of pulmonary edema. On
the other hand, these results may illuminate an important message that, for patients with mild-to-moderate ARAS (<70% diameter stenosis), according to clinical practice guidelines,
multifactorial medical therapy consisting of an angiotensin receptor-blocking agent, statin, antiplatelet therapy, smoking cessation, and management of diabetes is preferred. BP-LOWERING
EFFECT OF PTRA It should be noted that the BP-lowering effect was not defined as the primary outcome in the ASTRAL, STAR, CORAL, and RADAR trials. A meta-analysis of RCTs including 4 studies
(_n_ = 809) and comparison of balloon angioplasty with medical therapy in hypertensive patients with ARAS (>50% diameter stenosis) demonstrated a small improvement in diastolic BP in the
angioplasty group [mean difference (MD) −2.00 mmHg, 95% confidence interval (CI) −3.72 to −0.27; _p_ = 0.02], while a meta-analysis of 5 studies (_n_ = 1743) did not find a significant
improvement in systolic BP (MD −1.07 mmHg, 95% CI −3.45 to 1.30; _p_ = 0.38). On the other hand, the pooled results from 3 trials (_n_ = 1717) found that, at the end of follow-up, there was
a significant decrease in the number of antihypertensive drugs in the angioplasty group compared to the medical treatment group (MD −0.18, 95% CI −0.34 to −0.03; _p_ = 0.02) [16]. Therefore,
it would still be unwise to conclude that the BP-lowering effect of PTRA is insubstantial. A nonrandomized series of complex ARAS patients showed BP improvement by PTRA [19]; however, the
BP response in most of these previous trials was diagnosed based on office BP, and evidence concerning the effects of PTRA on out-of-office BP is limited. Eight nonrandomized studies with
unilateral or bilateral ARAS have provided information on the effectiveness of PTRA in lowering out-of-office BP (Table 2) [20,21,22,23,24,25,26,27]. One of these studies evaluated BP
response using home BP monitoring, and the others used ambulatory BP monitoring devices. Most of these studies were small and had a short duration of follow-up; however, studies including
>50 patients and having a relatively longer duration of follow-up have shown a certain level of BP reduction. RENOPROTECTIVE EFFECT OF PTRA Restoring blood flow to an ischemic kidney
beyond vascular occlusion or stenosis seems to provide an obvious means to restore kidney function; however, the strength of evidence for a renoprotective effect of PTRA is not so strong.
There have been several RCTs in which the renal outcome was defined as the primary outcome; however, the reported indexes of renal function were heterogeneous (Table 3) [5,6,7, 14, 28, 29].
The STAR trial, in which the primary endpoint was defined as a >20% decline in creatinine clearance, reported serum creatinine at either the end of the 2-year follow-up or when
participants reached the primary endpoint [6]. The ASTRAL trial reported the results of serum creatinine concentration for each year during the 5-year follow-up period [5]. The RADAR trial
reported changes in eGFR after 12 months [14], and the CORAL trial reported progressive renal insufficiency [7]. In all of these RCTs, there was no significant difference in serum
creatinine, creatinine clearance, worsening renal failure, or progressive renal insufficiency between the angioplasty and medical treatment groups (Table 3). One RCT found a significantly
higher prevalence of worsening renal function in the PTRA group, defined as an increase in serum creatinine level of ≥20% at the end of follow-up (_p_ < 0.001) [28]. A meta-analysis
including 3 trials (_n_ = 725) identified a statistically nonsignificant decrease in serum creatinine in the angioplasty group compared to the medical treatment group (MD −7.99 mmol/L, 95%
CI −22.6 to 6.62; _p_ = 0.28) [16]. A nonrandomized series of complex ARAS patients reported a renoprotective (or not worsening) effect of PTRA [30,31,32,33,34], and in some end-stage renal
disease patients, successful PTRA permitted discontinuation of hemodialysis treatment [35, 36]. These observations indicate that some patients certainly obtain a major clinical benefit from
successful PTRA; however, many other series of patients with various levels of renal dysfunction reported little change on average after PTRA [37]. In most of these previous trials, renal
function was diagnosed based on serum creatinine level or eGFR. It is recommended that renal function should be evaluated based not only on eGFR but also on albuminuria and/or proteinuria
[38]; however, there appears to be no solid data on the decrease in albuminuria or proteinuria after PTRA. A 1-year follow-up of 117 patients with successful PTRA demonstrated significant
improvement in morning home systolic BP (−12 ± 24 mmHg) and diastolic BP (−4 ± 10 mmHg) (both _p_ < 0.001) but no significant change in eGFR (46.2 ± 19.4 to 46.4 ± 10.2 ml/min/1.73 m2,
_p_ = 0.98), urinary albumin-to-creatinine ratio [3.2 (1.1–17.8) to 4.3 (1.0–26.4) mg/mmol, _p_ = 0.41], or protein/creatinine ratio [0 (0–44.8) to 10.9 (0–58.1) mg/mmol, _p_ = 0.08] [39].
CARDIOPROTECTIVE EFFECT OF PTRA There was low strength of evidence for cardiovascular risk reduction by PTRA [40]. In RCTs, the rates of cardiovascular events were similar between patients
treated with angioplasty and those treated with medical therapy [5, 7, 41]. However, as described above, patients who would likely benefit from PTRA, that is, those with very severe stenosis
and uncontrolled hypertension or recurrent sudden-onset pulmonary edema, were unlikely to be enrolled in these RCTs. There have been several observational trials suggesting that the
patients who are more likely to improve are those with cardiac destabilization syndromes after revascularization. Pulmonary edema was reported to be a risk factor for adverse outcomes in 467
ARAS patients, and compared to medical treatment, revascularization was associated with a reduced risk of death (hazard ratio 0.43, 95% CI, 0.20–0.91; _p_ = 0.01) [42]. In 48 refractory
hypertensive patients with severe ARAS (stenosis diameter >70%) who presented with ACS or decompensated heart failure, successful PTRA improved angina class without coronary
revascularization (3.1 ± 0.8 to 1.5 ± 0.9, _p_ < 0.05) in ACS patients and heart failure status in heart failure patients [New York Heart Association (NYHA) class, 2.8 ± 0.9 to 1.7 ± 1.2,
_p_ < 0.05] at 24 h after PTRA [43]. Similar findings were reported in 39 RAS patients (stenosis diameter >70%) with recurrent episodes of CHF and/or flash pulmonary edema, in which
successful PTRA resulted in a significant decrease in the number of hospital admissions due to heart failure as well as the severity of heart failure; NYHA class decreased from 2.9 ± 0.9 to
1.5 ± 0.9 [44]. Left ventricular (LV) hypertrophy is an established risk factor for worse outcomes, and a meta-analysis reported that ARAS patients had an increased likelihood of LV
hypertrophy [45]. LV hypertrophy may be reversed by treatment, and prospective studies in hypertensive cohorts have shown an association between regression of LV hypertrophy and reduced
cardiovascular complications [46, 47]. In both ARAS [45, 48] and FMD [48], PTRA produces a decrease in LV mass; however, in ARAS, in-treatment regression or continued absence of LV
hypertrophy seemed not to be associated with reduced risk of adverse cardiovascular outcomes [48]. BETTER APPROACH TO DETERMINING PTRA TREATMENT In most cases of RVH, the first goal of
treatment is to reduce BP and the morbidity and mortality associated with elevated BP. The second goal is to protect the hemodynamics and function of the kidneys. Finally, in the case of
uncontrolled heart failure, the goal is improvement of cardiac disturbance syndromes, including flash pulmonary edema. However, especially in ARAS, RVH is heterogeneous and progressive in
practice, and many RVH patients also have other comorbidities, such as essential hypertension, diabetes, or dyslipidemia, which adversely affect renal function and contribute to
therapy-resistant hypertension. Therefore, distinguishing between benign complications and actual RVH and ischemic nephropathy is difficult. However, there has been increasing evidence
concerning predictors that may be able to identify RAS patients who will benefit from PTRA. BEFORE PTRA BP EVALUATION Current international guidelines recommend out-of-office BP measurement
in clinical practice [12, 13, 49]. Out-of-office BP measurement using home BP or ambulatory BP monitoring seems to be crucial when PTRA treatment is being considered. Ambulatory BP
monitoring was performed in 191 RAS patients with ostial stenosis diameter ≥70% before and 1 year after PTRA and found that high baseline BP could be a predictor of better BP reduction [50].
This finding was confirmed in 72 ARAS patients (mean stenosis diameter, 78 ± 10%) with uncomplicated resistant hypertension defined as daytime ambulatory BP >135/85 mmHg, despite at
least 3 antihypertensive drugs including a diuretic. Baseline daytime ambulatory BP was 157 ± 16/82 ± 10 mmHg, and at a mean 2 months of follow-up after PTRA, BP had decreased by 14.0 ±
17.3/6.4 ± 8.7 mmHg, despite a decrease in the number of antihypertensive medications from 4.0 ± 1.0 to 3.6 ± 1.4. Furthermore, it was found that high baseline ambulatory daytime systolic BP
was an independent predictor of BP reduction (odds ratio 0.94, 95% CI: 0.90–0.99; _p_ = 0.025) [27]. These results suggest that a better BP response to PTRA can be expected in ARAS patients
with true resistant hypertension. Home BP was measured in 126 RVH patients (22% FMD patients) before and 1 year after PTRA, demonstrating that, although most patients were diagnosed with
uncontrolled morning home BP at baseline, at 1 year after PTRA, the prevalence of masked hypertension and uncontrolled hypertension was 32.5% and 6.4%, respectively [26]. This
disproportional serial reduction between office and home BP also supports the need for out-of-office BP measurements. ASSESSMENT OF RENAL FUNCTION RVH patients showing a rapid renal
functional decline are considered suitable candidates for PTRA treatment. Clear cutoff values to predict an improvement in renal function after PTRA have not been established, but the slope
of the reciprocal serum creatinine plot [51], annual rate of eGFR decline [52], and degree of serum creatinine elevation 6 months before PTRA [42, 53] were reported to be useful indices for
predicting the clinical course after PTRA. Before consideration of PTRA treatment, renal function should be evaluated based on both eGFR and albuminuria/proteinuria. PTRA has limited benefit
for patients with established long-term loss of eGFR, and decreased pretreatment eGFR and/or increased albuminuria/proteinuria is associated with an increased risk of worse outcomes after
PTRA [39, 54,55,56]. Both eGFR and albuminuria/proteinuria were evaluated in 139 ARAS patients before and 1 year after PTRA, and a significant divergent response of eGFR changes according to
the presence/absence of pretreatment albuminuria/proteinuria was found in those with a baseline eGFR of <45. In ARAS patients without albuminuria/proteinuria, PTRA treatment may have the
ability to stabilize or improve eGFR, and its improvement could be expected even in those with a pretreatment eGFR of <30 [39]. In a post hoc study of the CORAL trial, patients with low
pretreatment albuminuria in the angioplasty group showed better outcomes than those in the medical therapy group, but this was not observed in patients with high albuminuria [57]. These
results suggest that ARAS patients without pretreatment albuminuria/proteinuria may be ideal candidates for PTRA treatment. IMAGING TESTS: RENAL ULTRASONOGRAPHY AND RENAL RESISTIVE INDEX
(RI) The diagnosis of RVH is based on the demonstration of RAS and is therefore dependent upon imaging. Recent advances in noninvasive vascular imaging allow more frequent detection and
precise diagnostic assessment using Doppler ultrasonography, computed tomography angiography (CTA), and magnetic resonance angiography. Duplex Doppler renal ultrasonography is relatively
inexpensive and suitable as an initial imaging tool. This can provide both functional and structural assessment and is also suitable for serial studies to determine progression and/or
restenosis within the affected kidneys. Peak systolic velocity (PSV) >200 cm/s at the site of stenosis is associated with 95% sensitivity and 90% specificity for >50% stenosis, and a
ratio of renal artery to aortic PSV >3.5 has 92% sensitivity for >60% stenosis [10, 58]. It must be emphasized that, in the diagnosis of FMD, it is important to image the renal artery
in its entirety from the origin to the kidney parenchyma. As a practical matter, limitations of this technique include dependence upon operator skills and patient body build, and thus the
precision of testing may vary widely between institutions. Evaluation of kidney morphology should include not only kidney size but also anatomical structure because functional
characterization of the renal parenchyma distal to RAS may help predict revascularization outcomes. It has been suggested that renal function would not be expected to improve after
revascularization in cases of atrophic kidney size (<7 cm) [59], thinning of the renal parenchyma [50], or smaller parenchymal volume to radioisotope-assessed single-kidney GFR [60]. The
renal RI, measured by Doppler ultrasonography, is defined as [PSV minus minimum end-diastolic velocity]/PSV. In essential hypertensive patients, a higher RI obtained in proximal segmental
arteries has been reported to be correlated with the presence of hypertensive organ damage [61] and to predict poor cardiovascular and renal outcomes [62]. High RI (>0.8) has been
suggested to be associated with a worse renal outcome and with poor BP response to revascularization [63]. Several observational studies found similar findings, and low RI has been proposed
as a marker of likely benefit from PTRA [64, 65]. However, this has not been observed universally [39, 66, 67]. FMD OR ARAS The treatment strategy for RVH patients with ARAS differs from
that for patients with FMD. The BP response after PTRA is better in FMD [26], and cure for hypertension defined as removal of all antihypertensive therapy can be expected in considerable
cases. The likelihood of cure depends on factors such as the age of the patient and the duration and severity of hypertension [68]. Clinicians should consider PTRA, especially for younger
FMD subjects with hypertension, because without revascularization, many of them will require lifelong therapy, and PTRA may limit the need for ongoing medical therapy. PTRA/ANGIOGRAPHY
Digital subtraction angiography is the gold standard for characterizing RAS lesions and is usually combined with endovascular procedures, including dilation and stent placement. The precise
advantages and characteristics of various PTRA/angiography methods are beyond the scope of this review. Studies of human subjects with translesional pressure gradients indicate that an
aortic–renal pressure gradient of 10–20% is necessary to detect renin release [69], which corresponds to a translesional peak gradient of at least 20–25 mmHg and luminal stenosis of at least
70% [70]. Therefore, PTRA treatment has little effect on patients with mild-to-moderate RAS. A 99% stenotic lesion, or “string of beads sign,” may be easy to identify, but more commonly,
there is mild-to-moderate aorto-ostial stenosis, whose hemodynamic consequences are uncertain. In FMD, accurate visual assessment of the degree of stenosis is not possible with multifocal
lesions, and the presence of significant beading may not be associated with a hemodynamically significant gradient. Therefore, translesional pressure gradient measurement is recommended to
assess the hemodynamic significance of stenosis [71], and a pressure gradient >10% of the mean aortic pressure (i.e., resting distal renal artery pressure-to-aortic pressure ratio
<0.90) is proposed as the threshold for hemodynamically significant renal FMD for angioplasty [72]. In ARAS, based on expert consensus, angiographic stenosis diameter >70% is
considered severe or significant, and a stenosis diameter of 50–70% is considered moderate. For moderate-to-severe stenosis, confirmation of the hemodynamic severity of RAS is recommended
prior to stenting [10]. The translesional pressure gradient should be measured using a nonobstructive catheter or a 0.014-inch pressure wire. A resting or hyperemic translesional systolic
gradient ≥20 mmHg, a resting or hyperemic mean translesional gradient ≥10 mmHg, or a renal fractional flow reserve (RFFR) ≤0.8 can confirm hemodynamically severe RAS [10]. Hyperemia may be
induced with an intrarenal bolus of papaverine or dopamine [17]. In post hoc analyses, the mean hyperemic gradient, hyperemic systolic gradient, and RFFR have been reported to be able to
discriminate responders from nonresponders [73]. In general, the first-line revascularization technique in FMD-related RAS is PTRA without stenting, and in ARAS, it is PTRA with stenting. In
both FMD and ARAS, translesional pressure gradient measurement should be performed postangioplasty to confirm that the pressure gradient has disappeared. Atheroembolism typically occurs
during manipulation of the catheter while selecting and engaging the renal artery or while advancing the stent across the stenosis. As a preventive measure, embolic protection devices can be
used to prevent embolization during the placement of stents in the renal artery. Several observational studies report recovery of embolic debris and renal function stabilization after PTRA
[56]; however, its clinical value is under debate and has not been established. FOLLOW-UP AFTER PTRA/RESTENOSIS The initial clinical response to PTRA should be evaluated within 1–2 weeks
after the procedure. Antihypertensive medications should be reassessed at follow-up visits, and screening for restenosis should be considered in the setting of unexplained BP increase and/or
decline in renal function. In FMD, following renal angioplasty, prescription of 75–100 mg aspirin daily is recommended indefinitely, though some operators empirically prescribe a short
course of dual antiplatelet therapy (e.g., 4–6 weeks) [72]. In ARAS, dual therapy, such as aspirin and clopidogrel, seems to be the standard treatment after stenting and is commonly
administered for 4–6 weeks; however, dosing strategies for antiplatelet agents vary widely, and there are no prospective data on the comparative effectiveness of antiplatelet regimens after
PTRA. Restenosis and/or in-stent stenosis is one of the major complications that can occur at any time after renal PTRA, and accumulating experience shows that even angioplasty with stenting
was hampered by a high rate of restenosis [74]. The precise frequency of restenosis after angioplasty remains unclear and probably varies among institutions. Larger diameter target vessels
with a larger acute gain (poststent minimum lumen diameter) are reported to yield a lower restenosis rate than smaller diameter vessels with smaller acute gain [75]. The frequency of
restenosis after PTRA was investigated in 175 RVH patients (FMD 30.3%), and the rates of restenosis within a year and within 5 years were found to be 20% and 32%, respectively, with a much
higher rate in FMD than in ARAS [76]. In both FMD and ARAS, to detect findings suggestive of restenosis, surveillance with renal artery duplex ultrasound may include a study at the first
office visit postangioplasty (usually within 1 month) [72, 74]. In FMD, duplex imaging is recommended every 6 months for 24 months and then yearly [72]. It is necessary to adjust the
velocity parameters for a stented artery compared with a native vessel, as decreased compliance due to the stent will result in higher velocity [58]. In-stent stenosis of >70% can be
confirmed by PSV >395 cm/s [77]. If duplex imaging is inconclusive, CTA would be the next best test for determining stent patency. If ultrasound and CTA are still inconclusive and the
patient has recurrent clinical symptoms, angiography with hemodynamic confirmation of the severity of in-stent restenosis is indicated. CONCLUSIONS Physicians should recognize that restoring
renal blood flow is effective and critical for specific RAS patients. Current clinical guidelines indicate that reasonable candidates for PTRA are patients with hemodynamically significant
RAS with FMD with hypertension, refractory hypertension, rapid decline in renal function, or cardiac destabilization syndromes. As a practical matter, decisions for managing RAS patients
must be highly individualized. When high BP, reduced eGFR, or high vascular risk is present, it seems prudent to examine the renal hemodynamics and anatomy to diagnose the presence or
absence of RAS, its severity, whether bilateral or unilateral, and the relative size and functional characteristics of the kidney (Fig. 1). In some cases, the decision on therapeutic
strategy remains difficult, but clinicians must evaluate individual patients carefully to determine the relative merits of relying on medical therapy or adding PTRA. Further, it is essential
to consider ARAS as one aspect of atherosclerotic disease, which commonly progresses over a longer period of time, and some individuals with other comorbidities develop further
manifestations of high-grade stenosis and high-risk features. Antihypertensive drug therapy should be managed to achieve goal BP, which is undertaken as part of the decision as to whether
further consideration needs to be given to diagnostic and interventional procedures for RVH. Further investigation is required to identify RAS patients who demonstrably benefit from and
respond to PTRA. REFERENCES * Herrmann SM, Textor SC. Current concepts in the treatment of renovascular hypertension. Am J Hypertens. 2018;31:139–49. CAS PubMed Google Scholar *
Rocha-Singh K, Jaff MR, Rosenfield K, Aspire-Trial Investigators. Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2
study. J Am Coll Cardiol. 2005;46:776–83. PubMed Google Scholar * Weinberg I, Keyes MJ, Giri J, Rogers KR, Olin JW, White CJ, et al. Blood pressure response to renal artery stenting in 901
patients from five prospective multicenter FDA-approved trials. Catheter Cardiovasc Inter. 2014;83:603–9. Google Scholar * Jaff MR, Bates M, Sullivan T, Popma J, Gao X, Zaugg M, et al.
Significant reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: results from the Hercules trial. Catheter Cardiovasc Inter.
2012;80:343–50. Google Scholar * Astral Investigators, Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, et al. Revascularization versus medical therapy for renal-artery stenosis. N. Engl J
Med. 2009;361:1953–62. Google Scholar * Bax L, Woittiez AJ, Kouwenberg HJ, Mali WP, Buskens E, Beek FJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and
impaired renal function: a randomized trial. Ann Intern Med. 2009;150:840–8. PubMed Google Scholar * Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, et al. Stenting and
medical therapy for atherosclerotic renal-artery stenosis. N. Engl J Med. 2014;370:13–22. CAS PubMed Google Scholar * Raman G, Adam GP, Halladay CW, Langberg VN, Azodo IA, Balk EM.
Comparative effectiveness of management strategies for renal artery stenosis: an updated systematic review. Ann Intern Med. 2016;165:635–49. PubMed Google Scholar * Balk EM, Raman G, Adam
GP, Halladay CW, Langberg VN, Azodo IA, et al. _Renal Artery Stenosis Management Strategies: An Updated Comparative Effectiveness Review. Report No.: 16-EHC026-EF_. Rockville (MD): Agency
for Healthcare Research and Quality (US); 2016. * Klein AJ, Jaff MR, Gray BH, Aronow HD, Bersin RM, Diaz-Sandoval LJ, et al. SCAI appropriate use criteria for peripheral arterial
interventions: an update. Catheter Cardiovasc Inter. 2017;90:E90–110. Google Scholar * Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the
diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial
carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO), the Task Force for the Diagnosis and Treatment of
Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763–816. PubMed Google Scholar *
Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection,
evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines.
Hypertension. 2018;71:e13–15. CAS PubMed Google Scholar * Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension Guidelines for the
Management of Hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481. PubMed Google Scholar * Zeller T, Krankenberg H, Erglis A, Blessing E, Fuss T, Scheinert D, et al. A randomized,
multi-center, prospective study comparing best medical treatment versus best medical treatment plus renal artery stenting in patients with hemodynamically relevant atherosclerotic renal
artery stenosis (RADAR) - one-year results of a pre-maturely terminated study. Trials. 2017;18:380. PubMed PubMed Central Google Scholar * White CJ. The “chicken little” of renal stent
trials: the CORAL trial in perspective. JACC Cardiovasc Interv. 2014;7:111–3. PubMed Google Scholar * Jenks S, Yeoh SE, Conway BR. Balloon angioplasty, with and without stenting, versus
medical therapy for hypertensive patients with renal artery stenosis. Cochrane Database Syst Rev 2014:CD002944. * Prince M, Tafur JD, White CJ. When and how should we revascularize patients
with atherosclerotic renal artery stenosis? JACC Cardiovasc Interv. 2019;12:505–17. PubMed Google Scholar * Mishima E, Suzuki T, Ito S. Selection of patients for angioplasty for treatment
of atherosclerotic renovascular disease: predicting responsive patients. Am J Hypertens. 2020;33:391–401. PubMed Google Scholar * Chrysant SG. The current status of angioplasty of
atherosclerotic renal artery stenosis for the treatment of hypertension. J Clin Hypertens (Greenwich). 2013;15:694–8. Google Scholar * Plouin PF, Chatellier G, Darne B, Raynaud A. Blood
pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai multicentrique medicaments vs angioplastie (EMMA) study group. Hypertension.
1998;31:823–9. CAS PubMed Google Scholar * Mangiacapra F, Trana C, Sarno G, Davidavicius G, Protasiewicz M, Muller O, et al. Translesional pressure gradients to predict blood pressure
response after renal artery stenting in patients with renovascular hypertension. Circ Cardiovasc Interv. 2010;3:537–42. PubMed Google Scholar * Adel SM, Syeidian SM, Najafi M, Nourizadeh
M. Clinical efficacy of percutaneous renal revascularization with stent placement in hypertension among patients with atherosclerotic renovascular diseases. J Cardiovasc Dis Res.
2011;2:36–43. CAS PubMed PubMed Central Google Scholar * Protasiewicz M, Kadziela J, Poczatek K, Poreba R, Podgorski M, Derkacz A, et al. Renal artery stenosis in patients with resistant
hypertension. Am J Cardiol. 2013;112:1417–20. PubMed Google Scholar * Kadziela J, Januszewicz A, Prejbisz A, Michalowska I, Januszewicz M, Florczak E, et al. Prognostic value of renal
fractional flow reserve in blood pressure response after renal artery stenting (PREFER study). Cardiol J. 2013;20:418–22. PubMed Google Scholar * Jujo K, Saito K, Ishida I, Furuki Y, Ouchi
T, Kim A, et al. Efficacy of 24-hour blood pressure monitoring in evaluating response to percutaneous transluminal renal angioplasty. Circ J. 2016;80:1922–30. PubMed Google Scholar *
Iwashima Y, Fukuda T, Kusunoki H, Hayashi SI, Kishida M, Yoshihara F, et al. Effects of percutaneous transluminal renal angioplasty on office and home blood pressure and home blood pressure
variability in hypertensive patients with renal artery stenosis. Hypertension. 2017;69:109–17. CAS PubMed Google Scholar * Courand PY, Dinic M, Lorthioir A, Bobrie G, Grataloup C, Denarie
N, et al. Resistant hypertension and atherosclerotic renal artery stenosis: effects of angioplasty on ambulatory blood pressure. A retrospective uncontrolled single-center study.
Hypertension. 2019;74:1516–23. CAS PubMed Google Scholar * Ziakka S, Ursu M, Poulikakos D, Papadopoulos C, Karakasis F, Kaperonis N, et al. Predictive factors and therapeutic approach of
renovascular disease: four years’ follow-up. Ren Fail. 2008;30:965–70. PubMed Google Scholar * Marcantoni C, Zanoli L, Rastelli S, Tripepi G, Matalone M, Mangiafico S, et al. Effect of
renal artery stenting on left ventricular mass: a randomized clinical trial. Am J Kidney Dis. 2012;60:39–46. PubMed Google Scholar * Leertouwer TC, Gussenhoven EJ, Bosch JL, van Jaarsveld
BC, van Dijk LC, Deinum J, et al. Stent placement for renal arterial stenosis: where do we stand? A meta-analysis. Radiology. 2000;216:78–85. CAS PubMed Google Scholar * Hanzel G, Balon
H, Wong O, Soffer D, Lee DT, Safian RD. Prospective evaluation of aggressive medical therapy for atherosclerotic renal artery stenosis, with renal artery stenting reserved for previously
injured heart, brain, or kidney. Am J Cardiol. 2005;96:1322–7. PubMed Google Scholar * Arthurs Z, Starnes B, Cuadrado D, Sohn V, Cushner H, Andersen C. Renal artery stenting slows the rate
of renal function decline. J Vasc Surg. 2007;45:726–31. PubMed Google Scholar * Dichtel LE, Gurevich D, Rifkin B, Varma P, Concato J, Peixoto AJ. Renal artery revascularization in
patients with atherosclerotic renal artery stenosis and impaired renal function: conservative management versus renal artery stenting. Clin Nephrol. 2010;74:113–22. CAS PubMed Google
Scholar * Kane GC, Xu N, Mistrik E, Roubicek T, Stanson AW, Garovic VD. Renal artery revascularization improves heart failure control in patients with atherosclerotic renal artery stenosis.
Nephrol Dial Transpl. 2010;25:813–20. Google Scholar * Korsakas S, Mohaupt MG, Dinkel HP, Mahler F, Do DD, Voegele J, et al. Delay of dialysis in end-stage renal failure: prospective study
on percutaneous renal artery interventions. Kidney Int. 2004;65:251–8. CAS PubMed Google Scholar * Thatipelli M, Misra S, Johnson CM, Andrews JC, Stanson AW, Bjarnason H, et al. Renal
artery stent placement for restoration of renal function in hemodialysis recipients with renal artery stenosis. J Vasc Interv Radiol. 2008;19:1563–8. PubMed Google Scholar * Kashyap VS,
Sepulveda RN, Bena JF, Nally JV, Poggio ED, Greenberg RK, et al. The management of renal artery atherosclerosis for renal salvage: does stenting help? J Vasc Surg. 2007;45:101–8. PubMed
Google Scholar * Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney
Int Suppl. 2013;3:1–150. Google Scholar * Iwashima Y, Fukuda T, Horio T, Hayashi SI, Kusunoki H, Kishida M, et al. Association between renal function and outcomes after percutaneous
transluminal renal angioplasty in hypertensive patients with renal artery stenosis. J Hypertens. 2018;36:126–35. CAS PubMed Google Scholar * Riaz IB, Husnain M, Riaz H, Asawaeer M, Bilal
J, Pandit A, et al. Meta-analysis of revascularization versus medical therapy for atherosclerotic renal artery stenosis. Am J Cardiol. 2014;114:1116–23. PubMed Google Scholar * van
Jaarsveld BC, Krijnen P, Pieterman H, Derkx FH, Deinum J, Postma CT, et al. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery
Stenosis Intervention Cooperative Study Group. N. Engl J Med. 2000;342:1007–14. PubMed Google Scholar * Ritchie J, Green D, Chrysochou C, Chalmers N, Foley RN, Kalra PA. High-risk clinical
presentations in atherosclerotic renovascular disease: prognosis and response to renal artery revascularization. Am J Kidney Dis. 2014;63:186–97. PubMed Google Scholar * Khosla S, White
CJ, Collins TJ, Jenkins JS, Shaw D, Ramee SR. Effects of renal artery stent implantation in patients with renovascular hypertension presenting with unstable angina or congestive heart
failure. Am J Cardiol. 1997;80:363–6. CAS PubMed Google Scholar * Gray BH, Olin JW, Childs MB, Sullivan TM, Bacharach JM. Clinical benefit of renal artery angioplasty with stenting for
the control of recurrent and refractory congestive heart failure. Vasc Med. 2002;7:275–9. PubMed Google Scholar * Cuspidi C, Dell’Oro R, Sala C, Tadic M, Gherbesi E, Grassi G, et al. Renal
artery stenosis and left ventricular hypertrophy: an updated review and meta-analysis of echocardiographic studies. J Hypertens. 2017;35:2339–45. CAS PubMed Google Scholar * Devereux RB,
Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–6. CAS
PubMed Google Scholar * Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, et al. Prognostic significance of serial changes in left ventricular mass in essential
hypertension. Circulation. 1998;97:48–54. CAS PubMed Google Scholar * Iwashima Y, Fukuda T, Horio T, Kusunoki H, Hayashi SI, Kamide K, et al. Impact of percutaneous revascularization on
left ventricular mass and its relationship to outcome in hypertensive patients with renal artery stenosis. Am J Hypertens. 2020;33:570–80. PubMed Google Scholar * Williams B, Mancia G,
Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the
European Society of Cardiology and the European Society of Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Cardiology and the European
Society of Hypertension. J Hypertens. 2018;36:1953–2041. CAS PubMed Google Scholar * Zeller T, Frank U, Muller C, Burgelin K, Sinn L, Bestehorn HP, et al. Predictors of improved renal
function after percutaneous stent-supported angioplasty of severe atherosclerotic ostial renal artery stenosis. Circulation. 2003;108:2244–9. PubMed Google Scholar * Muray S, Martin M,
Amoedo ML, Garcia C, Jornet AR, Vera M, et al. Rapid decline in renal function reflects reversibility and predicts the outcome after angioplasty in renal artery stenosis. Am J Kidney Dis.
2002;39:60–6. PubMed Google Scholar * Vassallo D, Ritchie J, Green D, Chrysochou C, Kalra PA. The effect of revascularization in patients with anatomically significant atherosclerotic
renovascular disease presenting with high-risk clinical features. Nephrol Dial Transpl. 2018;33:497–506. CAS Google Scholar * Kim S, Kim MJ, Jeon J, Jang HR, Park KB, Huh W, et al. Effects
of percutaneous angioplasty on kidney function and blood pressure in patients with atherosclerotic renal artery stenosis. Kidney Res Clin Pract. 2019;38:336–46. PubMed PubMed Central
Google Scholar * Kennedy DJ, Colyer WR, Brewster PS, Ankenbrandt M, Burket MW, Nemeth AS, et al. Renal insufficiency as a predictor of adverse events and mortality after renal artery stent
placement. Am J Kidney Dis. 2003;42:926–35. PubMed Google Scholar * Zeller T, Muller C, Frank U, Burgelin K, Schwarzwalder U, Horn B, et al. Survival after stenting of severe
atherosclerotic ostial renal artery stenoses. J Endovasc Ther. 2003;10:539–45. PubMed Google Scholar * Textor SC, Misra S, Oderich GS. Percutaneous revascularization for ischemic
nephropathy: the past, present, and future. Kidney Int. 2013;83:28–40. CAS PubMed Google Scholar * Murphy TP, Cooper CJ, Pencina KM, D’Agostino R, Massaro J, Cutlip DE, et al.
Relationship of albuminuria and renal artery stent outcomes: results from the CORAL randomized clinical trial (Cardiovascular Outcomes with Renal Artery Lesions). Hypertension.
2016;68:1145–52. CAS PubMed Google Scholar * Chi YW, White CJ, Thornton S, Milani RV. Ultrasound velocity criteria for renal in-stent restenosis. J Vasc Surg. 2009;50:119–23. PubMed
Google Scholar * Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease
(lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for
Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Bbiology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing
committee to develop guidelines for the management of patients with peripheral arterial disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation;
National Heart, Lung, and Blood Institute; Society for Vascular Nursing; Transatlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–654. PubMed
Google Scholar * Chrysochou C, Green D, Ritchie J, Buckley DL, Kalra PA. Kidney volume to GFR ratio predicts functional improvement after revascularization in atheromatous renal artery
stenosis. PLoS ONE. 2017;12:e0177178. PubMed PubMed Central Google Scholar * Doi Y, Iwashima Y, Yoshihara F, Kamide K, Takata H, Fujii T, et al. Association of renal resistive index with
target organ damage in essential hypertension. Am J Hypertens. 2012;25:1292–8. CAS PubMed Google Scholar * Doi Y, Iwashima Y, Yoshihara F, Kamide K, Hayashi S, Kubota Y, et al. Renal
resistive index and cardiovascular and renal outcomes in essential hypertension. Hypertension. 2012;60:770–7. CAS PubMed Google Scholar * Radermacher J, Chavan A, Bleck J, Vitzthum A,
Stoess B, Gebel MJ, et al. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N. Engl J Med. 2001;344:410–7. CAS PubMed Google Scholar * Bruno RM,
Daghini E, Versari D, Sgro M, Sanna M, Venturini L, et al. Predictive role of renal resistive index for clinical outcome after revascularization in hypertensive patients with
atherosclerotic renal artery stenosis: a monocentric observational study. Cardiovasc Ultrasound. 2014;12:9. PubMed PubMed Central Google Scholar * Matayoshi T, Kamide K, Tanaka R, Fukuda
T, Horio T, Iwashima Y, et al. Factors associated with outcomes of percutaneous transluminal renal angioplasty in patients with renal artery stenosis: a retrospective analysis of 50
consecutive cases. Int J Hypertens 2018;2018:1952685. * Garcia-Criado A, Gilabert R, Nicolau C, Real MI, Muntana X, Blasco J, et al. Value of Doppler sonography for predicting clinical
outcome after renal artery revascularization in atherosclerotic renal artery stenosis. J Ultrasound Med. 2005;24:1641–7. PubMed Google Scholar * Crutchley TA, Pearce JD, Craven TE,
Stafford JM, Edwards MS, Hansen KJ. Clinical utility of the resistive index in atherosclerotic renovascular disease. J Vasc Surg. 2009;49:148–55. PubMed Google Scholar * Trinquart L,
Mounier-Vehier C, Sapoval M, Gagnon N, Plouin PF. Efficacy of revascularization for renal artery stenosis caused by fibromuscular dysplasia: a systematic review and meta-analysis.
Hypertension. 2010;56:525–32. CAS PubMed Google Scholar * De Bruyne B, Manoharan G, Pijls NH, Verhamme K, Madaric J, Bartunek J, et al. Assessment of renal artery stenosis severity by
pressure gradient measurements. J Am Coll Cardiol. 2006;48:1851–5. PubMed Google Scholar * Drieghe B, Madaric J, Sarno G, Manoharan G, Bartunek J, Heyndrickx GR, et al. Assessment of renal
artery stenosis: side-by-side comparison of angiography and duplex ultrasound with pressure gradient measurements. Eur Heart J. 2008;29:517–24. PubMed Google Scholar * Olin JW, Gornik HL,
Bacharach JM, Biller J, Fine LJ, Gray BH, et al. Fibromuscular dysplasia: state of the science and critical unanswered questions: a scientific statement from the American Heart Association.
Circulation. 2014;129:1048–78. PubMed Google Scholar * Gornik HL, Persu A, Adlam D, Aparicio LS, Azizi M, Boulanger M, et al. First international consensus on the diagnosis and management
of fibromuscular dysplasia. J Hypertens. 2019;37:229–52. CAS PubMed Google Scholar * van Brussel PM, van de Hoef TP, de Winter RJ, Vogt L, van den Born BJ. Hemodynamic measurements for
the selection of patients with renal artery stenosis: a systematic review. JACC Cardiovasc Interv. 2017;10:973–85. PubMed Google Scholar * Boateng FK, Greco BA. Renal artery stenosis:
prevalence of, risk factors for, and management of in-stent stenosis. Am J Kidney Dis. 2013;61:147–60. PubMed Google Scholar * Lederman RJ, Mendelsohn FO, Santos R, Phillips HR, Stack RS,
Crowley JJ. Primary renal artery stenting: characteristics and outcomes after 363 procedures. Am Heart J. 2001;142:314–23. CAS PubMed Google Scholar * Iwashima Y, Fukuda T, Yoshihara F,
Kusunoki H, Kishida M, Hayashi S, et al. Incidence and risk factors for restenosis, and its impact on blood pressure control after percutaneous transluminal renal angioplasty in hypertensive
patients with renal artery stenosis. J Hypertens. 2016;34:1407–15. CAS PubMed Google Scholar * Granata A, Fiorini F, Andrulli S, Logias F, Gallieni M, Romano G, et al. Doppler ultrasound
and renal artery stenosis: an overview. J Ultrasound. 2009;12:133–43. CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by a Japan
Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (C), Number 17K09743. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Nephrology and
Hypertension, Dokkyo Medical University, Mibu, Tochigi, Japan Yoshio Iwashima & Toshihiko Ishimitsu Authors * Yoshio Iwashima View author publications You can also search for this author
inPubMed Google Scholar * Toshihiko Ishimitsu View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Yoshio Iwashima.
ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Iwashima, Y., Ishimitsu, T. How
should we define appropriate patients for percutaneous transluminal renal angioplasty treatment?. _Hypertens Res_ 43, 1015–1027 (2020). https://doi.org/10.1038/s41440-020-0496-z Download
citation * Received: 22 May 2020 * Revised: 28 May 2020 * Accepted: 28 May 2020 * Published: 22 June 2020 * Issue Date: October 2020 * DOI: https://doi.org/10.1038/s41440-020-0496-z SHARE
THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * Atherosclerosis * Fibromuscular dysplasia * Percutaneous transluminal renal angioplasty * Renal
artery stenosis * Renovascular hypertension