Play all audios:
ABSTRACT MicroRNAs (miRNAs) associated with Argonaute proteins (AGOs) regulate gene expression in mammals. miRNA 3’ ends are subject to frequent sequence modifications, which have been
proposed to affect miRNA stability. However, the underlying mechanism is not well understood. Here, by genetic and biochemical studies as well as deep sequencing analyses, we find that AGO
mutations disrupting miRNA 3’ binding are sufficient to trigger extensive miRNA 3’ modifications in HEK293T cells and in cancer patients. Comparing these modifications in TUT4, TUT7 and
DIS3L2 knockout cells, we find that TUT7 is more robust than TUT4 in oligouridylating mature miRNAs, which in turn leads to their degradation by the DIS3L2 exonuclease. Our findings indicate
a decay machinery removing AGO-associated miRNAs with an exposed 3’ end. A set of endogenous miRNAs including miR-7, miR-222 and miR-769 are targeted by this machinery presumably due to
target-directed miRNA degradation. SIMILAR CONTENT BEING VIEWED BY OTHERS TENT2, TUT4, AND TUT7 SELECTIVELY REGULATE MIRNA SEQUENCE AND ABUNDANCE Article Open access 07 September 2022
REGULATION OF MICRORNA EXPRESSION BY THE ADAPTOR PROTEIN GRB2 Article Open access 16 June 2023 NON-CANONICAL RNA SUBSTRATES OF DROSHA LACK MANY OF THE CONSERVED FEATURES FOUND IN PRIMARY
MICRORNA STEM-LOOPS Article Open access 20 March 2024 INTRODUCTION MicroRNAs (miRNAs) are a class of small non-coding RNAs that serve as master regulators of gene expression in eukaryotic
cells1. miRNAs bind to Argonaute proteins (AGO), with their 5′ end embedded inside the AGO MID domain and the 3′ end docked at the AGO PAZ domain2. The AGO-miRNA complex forms the core of
the RNA-induced silencing complex (RISC). Through partial base-pairing, miRNAs guide the RISC to target mRNAs, downregulating their levels by translational repression and/or mRNA
degradation3. Most human mRNAs contain at least one functional miRNA target site, indicating that nearly all biological pathways are under miRNA regulation4. Not surprisingly, dysregulation
of miRNAs is associated with and potentially leads to human diseases5. While layers of regulation in miRNA biogenesis have been characterized6, little is known about miRNA turnover7. Due to
Argonaute protection, miRNAs are generally stable in cells, with half-lives ranging from hours to days8,9,10,11. Therefore, active decay is critical in situations that require rapid changes
in miRNA function12. Tudor-SN cleaves AGO-bound miRNAs containing CA and UA dinucleotides, playing an important role in regulating cell cycle transition13. Another mechanism to achieve
miRNA-specific decay is target-directed miRNA degradation (TDMD), in which extensively paired targets induce miRNA turnover14. TDMD was initially described to be triggered by artificial
targets as well as viral RNAs15,16. Recently, endogenous transcripts such as lncRNA CYRANO and _NREP_ mRNA were shown to down-regulate miR-7 (ref. 17) and miR-29 (ref. 18), respectively, via
TDMD, indicating that TDMD could be a general mechanism regulating miRNA stability. miRNAs are thought to be degraded from the 3′ end. During TDMD, extensive pairing between miRNA and
targets promotes the dislocation of the 3′ end of miRNA from AGO binding and makes it accessible to enzymatic modifications19. As a result, TDMD-induced miRNA turnover is often accompanied
by elevated levels of miRNA 3′ isoforms (3′ isomiRs)14,15. A set of terminal nucleotidyltransferases (TENTs), including TUT4 (ZCCHC11/TENT3A/PAPD3) and TUT7 (ZCCHC6/TENT3B/PAPD6) are
responsible for adding non-templated nucleotides to the 3′ ends of miRNAs (tailing), whereas 3′–5′ exonucleases shorten the miRNAs by removing nucleotides from the 3′ end (trimming)20,21,22.
3′ uridylated or adenylated isomiRs have different stabilities compared to the cognate miRNAs23,24,25 and plant miRNAs uridylated by HESO1 and URT1 at the 3′ termini are removed by 3′–5′
exonucleases26,27. However, it remains unclear how the stability of miRNAs is regulated by 3′ modifications in animals. Here, we investigate how 3′ modifications lead to miRNA decay by
generating mutations in the AGO PAZ domain. These mutations are sufficient to trigger extensive miRNA 3′ end modifications in cultured cells and in vivo, suggesting a machinery monitoring
the status of the miRNA 3′ end. We find that these AGO-bound miRNAs with exposed 3′ ends are oligouridylated by both TUT4 and TUT7 and subsequently degraded by DIS3L2. Interestingly,
abolishing oligo-tailing resulted in elevated trimming, suggesting that a tailing-independent trimming process functions redundantly in removing AGO-bound miRNAs with an exposed 3′ end. We
provide evidence that a set of endogenous miRNAs, including miR-7, miR-222, and miR-769, which are likely under regulation of TDMD, are targeted by the TUT-DIS3L2 machinery in HEK293T cells.
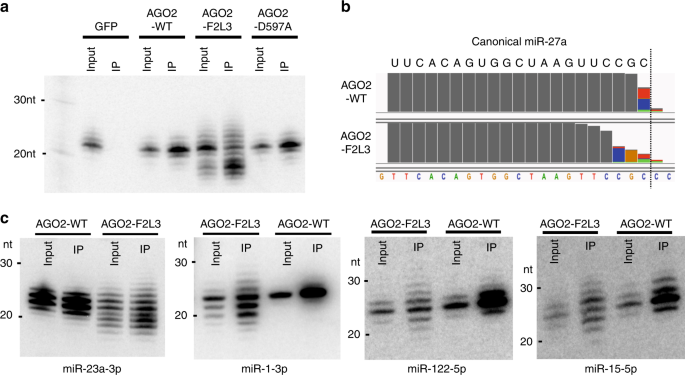
RESULTS DISRUPTING THE AGO PAZ DOMAIN PROMOTES MIRNA 3′ MODIFICATION We sought to test whether dislocating the miRNA 3′ end from AGO is sufficient to promote 3′ modifications. To this end,
we created an AGO2 mutant (AGO2-F2L3) containing two point mutations at the core of the PAZ domain binding pocket: F294A and L339A28. We co-expressed FLAG-tagged AGO2-F2L3 in HEK293T cells
with miR-27a, a miRNA known to be regulated by TDMD during viral infection16. Mature miR-27a associated with AGO2 mutant was pulled-down via immunoprecipitation and detected by Northern
blot. Results were AGO-specific since we detected no signal in the GFP pull-down control. We observed reduced miR-27a levels as well as extensive isomiRs with different electrophoretic
mobilities in the pull-downs of the PAZ mutant (AGO2-F2L3) but not in those of wild-type AGO2 (AGO2-WT) or a version of AGO2 mutated at the catalytic center (AGO2-D597A) (Fig. 1a). Deep
sequencing analyses confirmed that the aberrantly sized RNAs consisted of miR-27a of variable lengths (Supplementary Fig. 1a). Mapping reads to the genome sequence revealed that those
miR-27a isomiRs were a result of 3′ modifications, consisting of 3′ trimming, tailing or both (Fig. 1b, Supplementary Fig. 1b). Consistent with previous studies of miRNA 3′
modifications19,29,30, non-templated nucleotides added to the 3′ end of miR-27a were mainly U or A (Fig. 1b, Supplementary Fig. 1b). To rule out the possibility that these 3′ modifications
occurred on pre-miRNAs and were inherited by mature miRNAs, we repeated the same experiment with a synthetic miR-27a duplex, which is loaded to AGO2 directly (Supplementary Fig. 1c). We
observed extensive 3′ tailing and trimming with the guide strand miR-27a but not with the passenger strand (Supplementary Fig. 1d). Because the former but not the latter is retained in
mature RISC (Supplementary Fig. 1c), this result indicates that the 3′ modifications occurred on the mature miR-27a. Co-expressing AGO2-F2L3 with two additional 3p miRNAs (miR-23a and miR-1)
and two additional 5p miRNAs (miR-122 and miR-15) generated similar results (Fig. 1c). Since the 3′ end of 5p miRNAs is unavailable in the pre-miRNA (Supplementary Fig. 1c), these results
confirmed that the AGO PAZ mutation promotes modifications on AGO-bound mature miRNAs. Consistent with a recent report19, these results indicate that releasing the 3′ end from the PAZ domain
is sufficient to induce 3′ modifications. AGO PAZ mutants thus provide a unique platform for studying the 3′ modifications of miRNAs. ENDOGENOUS MIRNAS WITH EXPOSED 3′ ENDS UNDERGO
MODIFICATION Next, we deep-sequenced endogenous miRNAs in the pull-downs of either AGO2-WT or AGO2-F2L3. We observed increased 3′ modification of miRNAs associated with AGO2-F2L3 compared to
those associated with AGO2-WT (Fig. 2a), suggesting that the PAZ domain protects the 3′ terminus of AGO-bound miRNAs from being modified. Given that this phenomenon occurs for nearly all
miRNAs, the underlying machinery is unlikely to be sequence-specific. 5p miRNAs and 3p miRNAs had a similar degree of 3′ modification (Fig. 2b), indicating that the majority of these
modifications occur at the level of mature miRNA. These 3′ modifications mainly consisted of trimming (Fig. 2c, Supplementary Fig. 2a). As a result, shorter isomiRs were enriched in the
pull-downs of the AGO2 PAZ mutant (Fig. 2d). We validated these observations by Northern blot analyses of three abundant endogenous miRNAs (miR-10b, miR-7, and miR-148a) (Fig. 2e). Compared
to the results obtained with overexpressed miRNAs (Fig. 1), the apparent lack of 3′ tailing suggested that tailed isomiRs of endogenous miRNAs are efficiently removed in cells. To extend our
conclusion beyond cultured cells and artificially introduced AGO2 PAZ mutations, we took advantage of the datasets deposited in The Cancer Genome Atlas (TCGA) where various mutations in AGO
are documented and the corresponding miRNA sequencing data are available. miRNA 3′ modifications were more prevalent in a patient bearing an AGO2 mutation at the PAZ 3′ binding pocket
(P295L) than that in patients with AGO2 synonymous or missense mutations at other regions of AGO2 (Fig. 2f, Supplementary Fig. 2b). A similar result was obtained in a patient with another
AGO2 mutation in the PAZ 3′ binding pocket (R315M) (Supplementary Fig. 2b, c), but not in a patient with a mutation outside (E299K) (Supplementary Fig. 2b, d), indicating that the increased
3′ modifications are specific to the miRNA 3′ end dislocation. A mutation at the 3′ binding pocket of AGO1 (F310L) led to a similar result (Supplementary Fig. 2e, f), demonstrating that 3′
modifications are not limited to AGO2-bound miRNAs. Taken together, our results suggest a cellular machinery surveilling the 3′ end of AGO-bound miRNAs. Once exposed, 3′ ends of miRNAs are
subjected to extensive sequence modifications. TUT4 AND TUT7 OLIGOURIDYLATE THE 3′ END OF MATURE MIRNAS By taking advantage of the AGO PAZ mutant, we sought to identify the enzymes
responsible for tailing mature miRNAs. To facilitate detection of the tailed products, we generated a truncated form of AGO2 with a complete PAZ domain deletion (AGO2-ΔPAZ). We reasoned that
AGO2-ΔPAZ would make the 3′ end of miRNAs fully exposed and therefore increase the robustness of tailing. Indeed, we observed extensive tailing by Northern blot when miR-27a was
co-expressed with AGO2-ΔPAZ. We detected a ladder of bands with sizes of around 25–40 nt using a probe against mature miR-27a sequence (Fig. 3a), but not with probes against the miR-27a
passenger strand or loop sequence (Supplementary Fig. 3a), confirming that these bands were mature miR-27a with long tails. To further characterize these tailed products, we deep sequenced
small RNAs in the pull-downs of AGO2-ΔPAZ. Despite exhaustive efforts, we failed to clone the long-tailed isomiRs using conventional methods. We therefore increased the incubation time of 3′
adapter ligation during library preparation (see “Methods” for details). This procedure, which enabled the detection of isomiRs with long-tails, also led to nonspecific ligations between
miR-27a and various RNA fragments in cells, resulting in a high background of nonspecific tail sequences. To distinguish tailed isomiRs from noise, we compared the sequencing results of
AGO2-ΔPAZ-IP to those of AGO2-WT-IP, where long-tailed isomiRs were absent in the Northern blots (Fig. 3a). We reasoned that tail sequences detected in the AGO2-WT-IP represent the
background whereas sequences enriched in the AGO2-ΔPAZ-IP are derived from authentic miR-27a tails. Using this method, we found that U and, to a much lesser extent, A, but not G or C, were
enriched in the miR-27a oligo-tails (Fig. 3b, Supplementary Fig. 3b). The same experiment and analysis performed with miR-134-5p generated a similar result (Supplementary Fig. 3c),
indicating that long-tail isomiRs are largely a result of oligouridylation. TUT4 and TUT7 oligouridylate a set of pre-miRNAs to regulate their biogenesis31,32,33. We and others also showed
that TUT4 and TUT7 can uridylate mature miRNAs in vitro and in vivo34,35. Taking advantage of a previously established TUT4 and TUT7 double knockout (TUT4/7 DKO) HEK293T cell line35, we
tested whether TUT4 and TUT7 are the enzymes responsible for the robust oligouridylation of AGO-bound miRNAs with an exposed 3′ end. The accumulation of long-tailed miR-27a isomiRs resulting
from co-expression of AGO2-ΔPAZ was abolished in the TUT4/7 DKO cells (Fig. 3c “GFP lane”). This was rescued by ectopic expression of either TUT4 or TUT7 (Fig. 3c). Oligo-tailing of miR-27a
resulting from the co-expression of AGO2-F2L3, although less robust than that resulting from co-expression of AGO2-ΔPAZ, was impacted by TUT4 and TUT7 in a similar manner (Fig. 3d). The
same experiment performed with miR-23a generated a similar result (Supplementary Fig. 3d). Together, these results indicate that TUT4 and TUT7 are the major enzymes oligouridylating
AGO-bound miRNAs once their 3′ ends are exposed. TUT7 ASSOCIATES WITH ARGONAUTE PROTEINS Although expressing either TUT4 or TUT7 rescued oligouridylation of miRNAs in TUT4/7 DKO cells,
TUT7-mediated tails were substantially longer (Fig. 3c, d, Supplementary Fig. 3d), suggesting that TUT7 is more robust. To test this, we knocked out TUT4 and TUT7 individually in HEK293T
cells, confirming loss of their expression by Western blot (Supplementary Fig. 4a). While knocking out TUT7 diminished the long tails of miR-27a associated with the AGO2-ΔPAZ, depletion of
TUT4 had marginal impact on the oligouridylation of miR-27a (Fig. 4a). We observed a similar pattern with AGO2-F2L3-induced tails (Supplementary Fig. 4b) as well as oligouridylation of
miR-23a (Supplementary Fig. 4c), demonstrating that TUT7 is more robust than TUT4 in oligouridylating miRNAs with an exposed 3′ end. TUT4 and TUT7 have a similar potency in uridylation of
naked miRNA in vitro34. It is also known that their oligouridylation activity is increased when the enzymes are anchored to the substrate pre-let-7 via a bridge protein LIN2836,37. We
therefore hypothesized that the observed superior oligouridylation activity of TUT7 is due to its stronger association with the RISC. Indeed, we detected endogenous TUT7 but not TUT4 in the
immunoprecipitation pull-downs of FLAG-tagged AGO2-WT, AGO2-F2L3, and AGO2-ΔPAZ, but not in the pull-downs of FLAG-GFP with or without RNase treatment (Fig. 4b), indicating that TUT7
interacts with AGO2 in an RNA-independent manner. To confirm that the observed interaction was not due to overexpression of AGO2, we performed the reverse co-immunoprecipitation assay in
HEK392T cells without ectopic expression of either AGO or TUT. Supporting our conclusion, AGO1 and AGO2 were detected in the pull-downs of TUT7 but not in those of TUT4 (Fig. 4c). However,
given the limitations in the sensitivity of our assay, we cannot rule out the possibility that TUT4 also interacts with AGOs in cells. Only a small fraction of TUT7 and AGO2 were in complex
with each other (Fig. 4b, c), which is expected since TUT7 targets many other RNAs22 and only those AGO2-associated miRNAs with accessible 3′ ends are TUT7 targets. DIS3L2 DEGRADES
OLIGOURIDYLATED MATURE MIRNAS DIS3L2 removes pre-miRNAs oligouridylated by TUT4 and TUT738. To test whether DIS3L2 plays a similar role in degrading oligouridylated mature miRNAs, we
generated a HEK293T DIS3L2 KO cell line using CRISPR–Cas9 and confirmed loss of DIS3L2 expression by Western blot (Supplementary Fig. 5a). Upon co-expression of miR-27a with AGO2-ΔPAZ in
these cells, oligouridylated forms of miR-27a accumulated that were increased in length and intensity compared to WT cells (Fig. 5a). Long-tailed miR-27a isomiRs that were absent when
co-expressed with AGO2-F2L3 in WT cells became detectable in DIS3L2-KO cells (Fig. 5a). Deep sequencing analysis confirmed that the long miR-27a tails that accumulate upon DIS3L2 depletion
were due to oligouridylation (Fig. 5b, Supplementary Fig. 5b). Together, these results strongly suggest that oligouridylated miR-27a was targeted by DIS3L2 for degradation. Long-tailed
miR-27a isomiRs were absent in both WT and DIS3L2-KO cells when miR-27a was co-expressed with AGO2-WT (Fig. 5a, b, Supplementary Fig. 5b), confirming that an exposed 3′ end is required for a
miRNA to be oligouridylated. The same experiments performed with miR-23a-3p and miR-134-5p generated similar results (Supplementary Fig. 5c, d), suggesting that DIS3L2 degrades
oligouridylated mature miRNAs regardless of their sequence. Given that DIS3L2 constantly removes oligouridylated miRNAs in cells, we revisited the characteristics of miRNA tailing enzymes
without the interference of DIS3L2. To avoid having to knockout DIS3L2 in various TUT KO cell lines, we repressed DIS3L2 activity by co-expressing its catalytic-dead mutant (DIS3L2-CD-mut),
which functions in a dominant-negative manner39,40. DIS3L2 inhibition resulted in the detection of long U-tails on miR-27a in TUT7 KO cells (Fig. 5c, d, Supplementary Fig. 5e), indicating
that enzymes other than TUT7 contribute to oligouridylation of miRNAs. Further depletion of TUT4 (comparing TUT7 KO with TUT4/7 DKO) reduced the oligouridylation (Fig. 5c, d, Supplementary
Fig. 5e), demonstrating the contribution of TUT4. The fact that oligouridylated miR-27a isomiRs were still detectable in TUT4/7 DKO cells suggests that additional enzymes can carry out
oligouridylation (Fig. 5c), demonstrating extensive redundancy in the enzymes that tail AGO-bound miRNAs in cells. These conclusions were further supported by experiments performed with
AGO2-F2L3 (Supplementary Fig. 5f) and miR-23a (Supplementary Fig. 5g). To test whether DIS3L2 removes oligouridylated endogenous miRNAs, we transfected AGO2-WT, AGO2-F2L3, and AGO2-ΔPAZ in
wild-type cells or the corresponding DIS3L2 KO cells, and then performed immunoprecipitation. We analyzed endogenous miRNAs in the pull-downs by deep sequencing. Consistent with the idea
that the 3′ ends of miRNAs are protected by the AGO2-WT and become accessible when associated with AGO2 PAZ mutants, we observed accumulation of oligo-U-tails in the latter, but not in the
former, upon DIS3L2 depletion (Fig. 5e, Supplementary Fig. 5h). Together, these results support a model in which AGO-bound miRNAs with an exposed 3′ end are oligouridylated and subsequently
removed by DIS3L2. REDUNDANT MIRNA DECAY VIA TAILING-INDEPENDENT 3′ TRIMMING Abolishing oligouridylation by depleting TUT4 and TUT7 led to increased trimming (Fig. 3c, d, Supplementary Fig.
3d). Consistent with the idea that TUT7 is more robust in tailing mature miRNAs, knocking out TUT7 by itself was sufficient to trigger the accumulation of trimmed isomiRs (Fig. 4a,
Supplementary Fig. 4c), and this was rescued by the expression of TUT7 (Fig. 3c, d, Supplementary Fig. 3c, d). These results suggest that tailing enzymes compete with trimming enzyme(s) in
accessing the 3′ end of miRNAs. Depleting DIS3L2 or inhibiting DIS3L2 activity had no impact on the intensity of these trimmed isomiRs (Fig. 5a, Supplementary Fig. 5f, g). These results
indicate that the trimming process is likely independent of DIS3L2, which is consistent with the observation that DIS3L2 prefers oligouridylated substrates38,41. Blocking oligouridylation
had marginal effects on the level of miRNAs without 3′ modifications [see intensity of miRNA bands of canonical size (Figs. 3c and 4a, Supplementary Figs. 3d and 4b, c)], supporting a model
in which 3′ oligouridylation and 3′ trimming function redundantly to degrade AGO-bound miRNAs. Next, we deep sequenced endogenous miRNAs associated with AGO PAZ mutants in wild-type and
TUT4/7 DKO cells. Although blocking TUT4 and TUT7 impaired oligouridylation, it had no detectable effect on the overall oligo-tailing (uridylation and adenylation) of endogenous miRNAs
(Supplementary Fig. 5i), partially due to the fact that DIS3L2 constantly removes oligouridylated miRNAs. This also demonstrates the redundant contributions of other tailing enzymes.
Nonetheless, knocking out TUT4 and TUT7 led to a subtle increase in 3′ trimming (Supplementary Fig. 5j). Together, these results suggest that, besides the TUT-DIS3L2 machinery, a
tailing-independent trimming process functions redundantly in degrading AGO-bound mature miRNAs from the 3′ end. TUT-DIS3L2 IS IMPLICATED IN BUT NOT REQUIRED FOR TDMD To identify endogenous
miRNAs targeted by the TUT-DIS3L2 machinery, we sequenced miRNAs in wild-type and DIS3L2 KO cells and compared the tail composition for each miRNA. Upon DIS3L2 depletion, the average numbers
of U in tails increased for miR-7-5p, miR-222-3p, and miR-769-5p, among others (Fig. 6a). Further analyses confirmed that only oligo-U-tails and, to a much lesser extent, oligo-A-tails
accumulated (Fig. 6b, Supplementary Fig. 6a). Knocking out TUT4 and TUT7 had a marginal impact on the oligouridylation of miR-222-3p and miR-769-5p and no observable effect on miR-7-5p (Fig.
6b), suggesting that the majority of isomiRs oligouridylated by TUT4 and TUT7 are absent in wild-type cells, presumably removed by DIS3L2. These results were validated by Northern blot.
Extensive tailed-isomiRs of endogenous miR-7-5p, miR-222-3p and miR-769-5p accumulated in DIS3L2 KO cells (Fig. 6c). As expected, knocking out TUT4 and TUT7 together with DIS3L2 KO abolished
the extensive tailed-isomiRs (Fig. 6c), confirming that TUT4 and TUT7 are the main enzymes uridylating mature miRNAs. As a control, miR-21-5p did not gain U-tails upon DIS3L2 depletion
(Fig. 6a, c). Together, these results support a model in which most endogenous miRNAs have their 3′ ends protected by the AGOs whereas a subset of miRNAs, including miR-7-5p, miR-222-3p, and
miR-769-5p, have their 3′ ends exposed and are targeted by the TUT-DIS3L2 machinery. It is possible that miR-7-5p, miR-222-3p, and miR-769-5p have their 3′ ends exposed because they are
under active TDMD regulation in HEK293T cells. To test this, we sought to identify potential endogenous TDMD triggers. These triggers should be relatively abundant transcripts that contain
target sites with the potential to extensively base-pair with the corresponding miRNA42. We used the calculated binding energy between miRNA and its predicted target site (seed-paired) as an
indicator of the extent of base-pairing and measured the expression level of endogenous transcripts by RNA-seq. Analysis of miR-7-5p identified a known TDMD trigger—lncRNA CYRANO17
(Supplementary Fig. 6b), validating this approach. While many potential TDMD triggers were identified for miR-222-3p and miR-769-5p, few were found for other highly expressed miRNAs such as
miR-21-5p, miR-10a-5p, and miR-148a-3p (Supplementary Fig. 6b). This could explain why miR-7-5p, miR-222-3p, and miR-769-5p were targeted by the TUT-DIS3L2 machinery in HEK293T cells.
Consistent with this idea, knocking down CYRANO using two independent siRNAs (Supplementary Fig. 6c) resulted in upregulation of miR-7 and reduced 3′ tailing and trimming, whereas
overexpressing CYRANO, but not CYRANO with a mutated miR-7 binding site (CYRANO-mut) caused the opposite (Fig. 6d). Deep sequencing these samples confirmed that oligo-U tails but not oligo-C
or oligo-G tails correlated with the TDMD effect (Supplementary Fig. 6d). Consistent with a previous study17, oligo-A tailed forms of miR-7-5p also accumulated during TDMD (Fig. 6b,
Supplementary Fig. 6d), suggesting that adenylation enzymes are also involved. Taken together, these results suggest that the TUT-DIS3L2 machinery is a part of the TDMD pathway. Although
miR-7-5p is targeted by the TUT-DIS3L2 machinery in HEK293T cells, miR-7-5p did not accumulate in cells depleted of TUT4/7 or DIS3L2 (Fig. 6c). Neither did miR-222-3p nor miR-769-5p (Fig.
6c). In fact, the level of miR-7-5p decreased in DIS3L2 KO cells, possibly due to an approximately threefold increase in CYRANO levels in DIS3L2 KO cells (Supplementary Fig. 6e) and/or an
indirect effect of DIS3L2 depletion. Supporting this idea, levels of both U6 snRNA and Tyr-tRNA, were also reduced in the DIS3L2 KO cells (Supplementary Fig. 6f). Overexpressing CYRANO
induced a similar degree of miR-7-5p decay in wild-type, TUT4/7 DKO and DIS3L2 KO cells (Fig. 6e). The reduction of canonical miR-7-5p was somewhat attenuated upon TUT4/7 or DIS3L2 depletion
based on the Northern blot quantification (Supplementary Fig. 6g). Nonetheless, these results suggest that the TUT-DIS3L2 machinery is not essential for TDMD. It is possible that
exonucleases other than DIS3L2 function in parallel during TDMD by a 3′ trimming process that is independent of uridylation. To test this, we knocked down PARN, an A-tail-specific
exonuclease, or EXOSC3, a key component of the cytoplasmic exosome complex, in both HEK293T cells and DIS3L2 KO cells > 90% by siRNAs (Supplementary Fig. 6h). Consistent with a previous
study17, knocking down PARN had marginal, if any, impact on the level of miR-7-5p. On the other hand, depletion of EXOSC3 by two independent siRNA sequences led to accumulation of trimmed
miR-7-5p isoforms with or without DIS3L2 (Supplementary Fig. 6i). This effect is specific to miR-7-5p which is under active TDMD regulation (Supplementary Fig. 6i), suggesting that the
exosome functions independently of the TUT-DIS3L2 machinery in TDMD. Quantification of Northern blot results of three biological replicates revealed a consistent but subtle increase of
miR-7-5p relative to the miR-21-5p control upon exosome knocking down (Supplementary Fig. 6j), suggesting a redundant role of other exonuclease(s) besides DIS3L2 and exosome. DISCUSSION
Although there is increasing evidence that 3′ non-templated tails are involved in miRNA turnover7, the underlying mechanism remains elusive. Here, by creating AGO2 mutants carrying mutations
at the PAZ domain, we studied how miRNA 3′ end modifications lead to decay. We provide evidence that AGO-bound mature miRNAs with an exposed 3′ end undergo oligo-tailing by TENTs including
TUT4 and TUT7 and subsequent DIS3L2 degradation. Given that DIS3L2 prefers uridylated substrates38,41, our data explain why miRNA uridylation is associated with instability whereas
adenylation often stabilizes miRNAs7. Our results also suggest a tailing-independent 3′ trimming process which functions in parallel to degrade miRNAs, since blocking oligo-tailing or DIS3L2
function by itself does not abolish miRNA decay. Nonetheless, the finding of increased oligouridylated isomiRs in DIS3L2 knock-out cells reveals that a set of endogenous miRNAs are targeted
by the TUT-DIS3L2 machinery. Together, our findings support a model in which the stability of miRNAs is controlled, at least in part, by the accessibility of their 3′ ends. The TUT-DIS3L2
pathway, together with other nucleases, efficiently removes AGO-bound miRNAs with an accessible 3′ end (Fig. 7). In plants, the redundancy between uridylation and trimming in miRNA decay,
the AGO-TENT interaction, and the requirement of AGO for miRNA uridylation have been previously reported43,44,45,46. Our results both extend these observations and reveal that this is a
conserved mechanism for controlling miRNA stability in both plants and animals. Our findings also lay the foundation for future studies to identify additional factors involved in miRNA
decay. Uridylation by TENTs in concert with DIS3L2 regulates the stability of a wide range of RNAs. TUT4 and TUT7 were initially identified as regulators of miRNA biogenesis at the precursor
level31,32,33. Recently, TENTs-DIS3L2 has been identified as a cytoplasmic RNA surveillance pathway that degrades de-adenylated mRNAs47, defective pre-miRNAs48, mirtron-precursors49,50,
aberrant structured non-coding RNAs51,52,53, unprocessed tRNAs54, yeast Ago1-associated small RNAs55, and rRNAs56. Together with previous reports that TUT4 and TUT7 uridylate miRNAs34,35,57,
our findings establish this machinery in miRNA decay as well. The majority of miRNAs have their 3′ ends protected by AGOs, leaving only a small set of miRNAs targeted by DIS3L2 in HEK293T
cells. Therefore, the TUT-DIS3L2 machinery is unlikely to be a house-keeping pathway for miRNA turnover. Rather, it may function in situations that require rapid changes in miRNA abundance.
Among the 11 TENTs identified in mammals, TENT1 (TUT1) in addition to TUT4 and TUT7 is implicated in uridylation of mature miRNAs21,22. The residual uridylation observed in cells depleted of
TUT4 and TUT7 is likely due to TENT1 activity. In parallel, TENT2 (TUT2/GLD2/PAPD4), TENT4A, and TENT4B are involved in adenylation of miRNAs21,22. A recent report demonstrated that miRNAs
with a 3′ A-tail are also degraded by DIS3L258. These data indicate that miRNA 3′ modification is a complex and dynamic process and there are multiple mechanisms degrading miRNAs in
parallel. This extensive redundancy at the level of tailing, trimming and miRNA decay highlights the importance of controlling miRNA stability via 3′ modifications while presenting a
challenge in establishing the function of individual pathways. Future studies illustrating the specificity of various TENTs will aid our understanding of how these pathways are coordinated
to regulate miRNA turnover. Our findings implicate the TUT4/7-DIS3L2 machinery in TDMD, consistent with a previous report that DIS3L2 and TENT1 co-purify with TDMD targets59. However,
knocking out DIS3L2 or TUT4 and TUT7 does not abolish CYRANO-induced TDMD of miR-7 in HEK293T cells, suggesting that there are redundant pathways, possibly a uridylation-independent 3′
trimming process mediated by exonucleases other than DIS3L2. Supporting this idea, inhibition of exosome function led to a subtle attenuation of TDMD efficiency and accumulation of trimmed
forms of miR-7-5p isomiRs. It is possible that the exosome degrades trimmed isomiRs that disassociate from AGO. It is also possible that miRNAs are degraded by a means that is independent of
3′ modifications. Since TDMD of a given miRNA can be induced by multiple target sequences, it is intriguing to hypothesize that the underlying mechanism is dependent on the nature of its
trigger. Nearly all endogenous miRNAs are subjected to 3′ modifications20, indicating that their 3′ ends are accessible at some point. However, only a small set of miRNAs are targeted by the
TUT-DIS3L2 machinery. It is possible that a dislocated 3′ end, after being modified, falls back into the AGO PAZ domain, preventing further modifications. The TUT-DIS3L2 machinery is only
implicated when the miRNA 3′ end is exposed for a prolonged amount of time. We have identified several AGO mutations in the PAZ domain in cancer patients which are correlated with increased
levels of 3′ modifications. These mutations may contribute to cancer progression by altering the half-life of certain miRNAs. Likewise, dysregulation of miRNA stability may contribute to
phenotypes associated with TUT or DIS3L2 deficiency60. We demonstrated previously that 3′ mono-uridylation alters the way in which miRNAs interact with their targets35. Here, we find that 3′
oligouridylation leads to miRNA decay by DIS3L2. Future studies will address how various 3′ modifications and the resulting 3′ isomiRs coordinate in regulating miRNA function and stability.
METHODS PLASMIDS AND SIRNA For miRNA expression plasmids, the genomic sequence of miRNA and its flanking region (~250 bp on each side) was cloned into a CMV (Pol II) driven expression
vector. The AGO2-F2L3, AGO2-D597A, and AGO2-ΔPAZ plasmids were generated by mutagenesis of pIRESneo-FLAG/HA AGO2 (Addgene, #10822)61. The coding sequence of DIS3L2 was polymerase chain
reaction (PCR) amplified from a pool of HEK293T cDNAs and then cloned into pIRESneo-FLAG/HA at NheI and EcoRI sites using In-fusion HD kit (Clontech). The DIS3L2-CD-mut (catalytic-dead
mutant) plasmid was generated by point mutation of D391N on pIRESneo-FLAG/HA-DIS3L2 using Q5 Site-Directed Mutagenesis Kit (NEB). CYRANO and CYRANO-mut expression constructs were generated
by cloning the synthesized dsDNA oligos (gblocks, IDT) into the psiCHECK2 vector between XhoI and SpeI sites using In-fusion HD kit (Clontech). siRNAs against CYRANO were referenced from Kim
et al.62 and synthesized by IDT. siRNAs against EXOSC3 were a gift from Dr. Sandra Wolin’s Lab63. siRNAs against PARN were purchased from Dharmacon. Sequences of oligos and siRNA were
listed in Supplementary Table 1. CELL CULTURE AND TRANSFECTION HEK293T cells were purchased from ATCC (CRL-11268) and were maintained in high glucose DMEM (Thermo Fisher Scientific)
supplemented with 10% FB Essence (VWR), 1× GlutaMAX™-I (Thermo Fisher Scientific), 1× MEM Non-Essential Amino Acids (Thermo Fisher Scientific), and 100 U/ml penicillin and 100 μg/ml
streptomycin mixture (Thermo Fisher Scientific). For plasmid and siRNA transfection, Lipofectamine 3000 and Lipofectamine RNAiMAX were used, respectively, according to the manufacturer’s
protocols. TUT4 KO, TUT7 KO, and DIS3L2 KO cell lines were established by transfecting LentiCRISPR V2-sgRNA with puromycin resistant marker into HEK293T cells. DIS3L2-TUT4/7 TKO cell line
was established by transfecting DIS3L2KO cells with TUT4 and TUT7 LentiCRISPRV2-sgRNAs with hygromycin and zeocin resistant marker, respectively. Single colonies were picked and screened
after drug selection. WESTERN BLOT Total proteins were extracted by lysing the cells in the modRIPA buffer (10 mM Tris-Cl pH 7.0, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and 0.1% sodium
dodecyl sulfate (SDS)) supplemented with protease inhibitor cocktail (Roche). Proteins were resolved on 4–15% Mini-PROTEAN® TGX stain-free™ protein gels (Bio-Rad) and transferred onto a PVDF
membrane using Trans-Blot Turbo Transfer System (Bio-Rad) according to the manufacturer’s instructions. The primary antibodies used in this study were anti-DIS3L2 (1:1000, Sigma
#HPA035797), anti-ZCCHC11 (1:500, Proteintech, #18980-1-AP), anti-ZCCHC6 (1:2000, Proteintech, #25196-1-AP), anti-Tubulin (1:3000, Sigma, #T9026), anti-AGO1 (1:500, Wako, #015-22411), and
anti-AGO2 (1:500, Wako, #015-22031). The signals were developed with SuperSignal West Pico Chemiluminescent Substrate (Pierce) for strong signals or Immobilon Western Chemiluminescent HRP
Substrate (Millipore) for weak signals and imaged by the Chemidoc Touch Imaging System (Bio-Rad). NORTHERN BLOT Total RNA was extracted by using TRIzol reagent (Ambion). Twenty microgram
total RNA and a 32P-labeled Decade marker (Ambion) were loaded into 20% (w/v) acrylamide/8 M urea gels. After gel electrophoresis, RNAs were transferred onto Hybond-N membranes (Amersham
Pharmacia Biotech) using a semidry transfer apparatus, followed by either UV cross-linking using 1500J for detecting over-expressed miRNAs or EDC (1-ethyl-3-(3-dimethylaminopropyl)
carbodiimide)-mediated chemical cross-linking (Sigma) at 60 °C for 1 h for detecting endogenous miRNAs. 32P-labeled probes were hybridized with membrane overnight at 37 °C after
pre-hybridized with PerfectHyb™ Plus Hybridization Buffer (Sigma) at 37 °C for 10 min. After washing with 2× SSC plus 0.1% SDS buffer for 3 × 15 min at 37 °C, the membrane was exposed to an
Imaging Screen-K (Bio-Rad) overnight. Images were then analyzed by Typhoon Trio Imaging System (GE Healthcare). Northern blot results were quantified by Quantity One (Bio-Rad). The sequences
of probes used in this study were listed in Supplementary Table 1. RNA IMMUNOPRECIPITATION For isolating FLAG-tagged AGO2-bound miRNAs, one 10-cm dish of HEK293T cells transfected with
AGO2-WT, AGO2-F2L3, or AGO2-ΔPAZ, with or without miRNA expression vector, were lysed in 1 ml modRIPA buffer (10 mM Tris-Cl pH 7.0, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and 0.1% SDS)
supplemented with proteinase inhibitor cocktail (Roche). Cell lysates were incubated with 50 µl Anti-FLAG M2 Magnetic Beads (Sigma) at 4 °C overnight with rotation. For immunoprecipitation
of endogenous AGO2-bound RNAs, HEK293T, DIS3L2 KO, and TUT4/7 DKO cells were collected and lysed with NP-40 buffer (50 mM HEPES-KOH [pH 7.4], 150 mM KCl, 2 mM EDTA, 0.5 mM DTT, 0.5% NP-40,
complete EDTA-free protease inhibitor cocktail), 1 mM NaF, 5% [v/v] glycerol). The lysates were incubated with anti-AGO2 monoclonal antibody (Wako, #015-22031) conjugated to protein G
magnetic beads (Bio-Rad, #1614023) for 1 h at RT with rotation. After five washes with BC150 buffer (20 mM Tris-HCl (pH 8.0), 150 mM KCl, 0.2 mM EDTA, 10% glycerol) at room temperature, the
beads were lysed in 1 ml Trizol (Life Technologies) for RNA extraction. PROTEIN CO-IMMUNOPRECIPITATION (CO-IP) For FLAG co-IP, FLAG-tagged AGO2-WT, AGO2-F2L3, or AGO2-ΔPAZ were transfected
into HEK293T cells. Forty-eight hour after transfection, cells were lysed in modRIPA buffer. Cell lysates were cleared by centrifugation at 20,000_g_ for 15 min at 4 °C. Totally, 5%
supernatants were saved as input and the remaining was incubated with pre-washed Anti-FLAG M2 magnetic beads (Sigma #M8823) at 4 °C overnight. The beads were washed with TBS buffer (50 mM
Tris-Cl, PH 7.4, and 150 mM NaCl) for five times and then divided into two tubes. One tube was added with protein loading buffer (RNase−) and the other tube was treated with 1 µl RNase A
(Thermo Scientific #EN0531) and 1 µl RNase T1 (Thermo Scientific #EN0542) for 20 min at room temperature (RNase+). The IP samples were loaded to the protein gel as described above and
detected with anti-ZCCHC11 (1:500, Proteintech #18980-1-AP), anti-ZCCHC6 (1:2000, Proteintech #25196-1-AP), and anti-AGO2 (1:500, Wako, #015-22031). For the endogenous protein interaction,
HEK293T cells were collected and co-IP was performed using either anti-ZCCHC11 or anti-ZCCHC6 to pull down endogenous TUT4 or TUT7, treated with or without RNase A/T1, and then Western blot
was done to detect AGO1, AGO2, TUT4, and TUT7. REAL-TIME PCR Five microgram total RNA was used for reverse transcription by SuperScript IV reverse transcriptase (Thermo Fisher Scientific)
and random hexamers according to the manufacturer’s instructions. 4 µl diluted cDNA (1:8) was used for real-time qPCR using iQ SYBR green supermix (Bio-Rad) in 10 µl reaction. Data were
collected in CFX384 Touch Real-time PCR detection system (Bio-Rad). PCR primers used in this study were listed in Supplementary Table S1. SMALL RNA SEQUENCING Small RNA libraries were
constructed by NEBNext® small RNA library preparation kit (NEB, E7330) according to the manufacturer’s protocol with minor modifications. In particular, 1 μg total RNA or 300 ng of AGO2-IP
RNA was ligated to the 3′ adapter at a lower temperature (16 °C), with a higher PEG concentration (20%) and for a longer time (18 h). The cDNAs were then PCR amplified for 12–15 cycles, and
the amplified library was purified by running on a 6% (w/v) native acrylamide gel with a 20-bp ladder. The 140–160 bp fraction of the library was excised from the gel, and then purified by
ethanol precipitation. The small RNA library quality was assessed on the Agilent 2100 Bioanalyzer (Agilent), and the quantity was determined by Qubit dsDNA HS Assay (Life Technologies). Each
small RNA library was sequenced on an Illumina MiSeq platform (Illumina) with MiSeq® Reagent Kit v3-150 cycle (Illumina). ANALYSIS OF SMALL RNA SEQUENCING DATA AND TAIL COMPOSITION The
small RNA sequencing data were analyzed using an in-house pipeline. Briefly, adapters were removed, reads were mapped using Bowtie and visualized using IGV. More detailed study of the isomiR
profile was done using QuagmiR64. This software uses a unique algorithm to pull specific reads and aligns them against a consensus sequence in the middle of a miRNA, allowing mismatches on
the ends to capture 3′ isomiRs. The reports included tabulated analysis of miRNA expression, length, number of nucleotides trimmed and tail composition at individual read level. Customized R
scripts were used to calculate percentages of canonical miRNA (defined as the most abundant templated read) and 3′ isomiRs, as well as percentages of tailing and trimming. Long tail
composition was calculated by counting the number of non-templated nucleotides present in the tail of each isomiR read. Reads with equal number of non-templated nucleotides in the tail were
added together and cumulative distribution was calculated for all the oligo-tailed isomiRs going from ones with longer to shorter tails. Script of the R code used to generate this tail
composition analysis is available at GitHub. ANALYSIS OF ISOMIR PROFILES ON AGO1 AND AGO2 FROM TCGA Tumoral samples from TCGA bearing genomic mutations in either AGO1 or AGO2 leading to
missense and synonymous amino acid changes were identified from Genomic Data Commons Data Portal (accessed during May 2019). GDC uses combined reports from several variant callers (mutect2,
varscan, muse and somaticsniper). Selected Case ID were: P295L TCGA-53-A4EZ, R315M TCGA-HU-A4G8; and E299K TCGA-Z6-A8JE (AGO2), F310L TCGA-94-7033 (AGO1). The analysis of selected patient
samples was also performed using QuagmiR64, with a previous conversion of the bam files to fastq files by Picard Sam-to-Fastq, using Amazon cloud instances through the Seven Bridges Genomics
implementation of the NCI Cancer Genomics Cloud. Script of the R code used to analyze the impact of AGO mutations is available at GitHub. Mutations were plotted into the PDB structures of
AGO1 and AGO2 using pymol. BIOINFORMATIC PREDICTION OF TDMD TRIGGERS Prediction of target RNAs with extensive 3′ pairing with miRNAs that could induce the dislocation of the 3′ end of the
miRNAs from the PAZ domain was obtained by identifying RNAs with a 7mer seed from the list of human mRNA 3′UTRs (TargetScan7.2) and lncRNA (LNCipedia). Next, for each miRNA-RNA pair their
hybridization minimum free energy (MFE) was calculated using RNAduplex from the ViennaRNA Package 2.065. MFE for each miRNA-RNA hybrid was plotted against the abundance of the target RNA in
HEK293 cells35. Script of the R code used to predict TDMD triggers is available at GitHub. STATISTICS AND REPRODUCIBILITY Wilcoxon’s test (two-sided) for paired values were used to calculate
the _p_ values for the same group of miRNAs between two conditions or treatments. Otherwise, unpaired Student’s _t_ test (two-sided) was used for comparing two groups. Error bars displayed
on graphs represent the mean ± SEM of at least three independent experiments. All Northern blot and Western blot results showed here are representative results from at least three
independent experiments. REPORTING SUMMARY Further information on research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY The data
that support this study are available from the corresponding author upon reasonable request. Sequence data that support the findings of this study have been deposited in GEO with the
accession codes GSE139567 and GSE121327. The source data underlying Figs. 1a, 1c, 3a, 2a–d, f, 3b–d, 4a–c, 5a–e, 6a–e, and Supplementary Figs. 1d, 2a, d, f, 3a–d, 4a–c, 5a–j, 6a–j are
provided as a Source Data file, which is also available at Mendeley (https://doi.org/10.17632/s5hss3jw6k.2). CODE AVAILABILITY Scripts of the R code used in this study are available at
GitHub. Other code is available from the corresponding author upon reasonable request. REFERENCES * Bartel, D. P. Metazoan microRNAs. _Cell_ 173, 20–51 (2018). Article CAS PubMed PubMed
Central Google Scholar * Sheu-Gruttadauria, J. & MacRae, I. J. Structural foundations of RNA silencing by argonaute. _J. Mol. Biol._ 429, 2619–2639 (2017). Article CAS PubMed PubMed
Central Google Scholar * Gu, S. & Kay, M. A. How do miRNAs mediate translational repression? _Silence_ 1, 11 (2010). Article PubMed PubMed Central CAS Google Scholar * Friedman,
R. C., Farh, K. K.-H., Burge, C. B. & Bartel, D. P. Most mammalian mRNAs are conserved targets of microRNAs. _Genome Res._ 19, 92–105 (2009). Article CAS PubMed PubMed Central Google
Scholar * Lin, S. & Gregory, R. I. MicroRNA biogenesis pathways in cancer. _Nat. Rev. Cancer_ 15, 321–333 (2015). Article CAS PubMed PubMed Central Google Scholar * Treiber, T.,
Treiber, N. & Meister, G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. _Nat. Rev. Mol. Cell Biol._ 20, 5–20 (2019). Article CAS PubMed Google
Scholar * Rüegger, S. & Großhans, H. MicroRNA turnover: when, how, and why. _Trends Biochem. Sci._ 37, 436–446 (2012). Article PubMed CAS Google Scholar * Bail, S. et al.
Differential regulation of microRNA stability. _RNA_ 16, 1032–1039 (2010). Article CAS PubMed PubMed Central Google Scholar * Gantier, M. P. et al. Analysis of microRNA turnover in
mammalian cells following Dicer1 ablation. _Nucleic Acids Res._ 39, 5692–5703 (2011). Article CAS PubMed PubMed Central Google Scholar * Marzi, M. J. et al. Degradation dynamics of
microRNAs revealed by a novel pulse-chase approach. _Genome Res._ 26, 554–565 (2016). Article CAS PubMed PubMed Central Google Scholar * Guo, Y. et al. Characterization of the mammalian
miRNA turnover landscape. _Nucleic Acids Res._ 43, 2326–2341 (2015). Article ADS CAS PubMed PubMed Central Google Scholar * Krol, J. et al. Characterizing light-regulated retinal
microRNAs reveals rapid turnover as a common property of neuronal microRNAs. _Cell_ 141, 618–631 (2010). Article CAS PubMed Google Scholar * Elbarbary, R. A. et al. Tudor-SN-mediated
endonucleolytic decay of human cell microRNAs promotes G1/S phase transition. _Science_ 356, 859–862 (2017). Article ADS CAS PubMed PubMed Central Google Scholar * Fuchs Wightman, F.,
Giono, L. E., Fededa, J. P. & de la Mata, M. Target rnas strike back on micrornas. _Front. Genet._ 9, 435 (2018). Article PubMed PubMed Central CAS Google Scholar * Ameres, S. L. et
al. Target RNA-directed trimming and tailing of small silencing RNAs. _Science_ 328, 1534–1539 (2010). Article ADS CAS PubMed PubMed Central Google Scholar * Cazalla, D., Yario, T.
& Steitz, J. A. Down-regulation of a host microRNA by a Herpesvirus saimiri noncoding RNA. _Science_ 328, 1563–1566 (2010). Article ADS CAS PubMed PubMed Central Google Scholar *
Kleaveland, B., Shi, C. Y., Stefano, J. & Bartel, D. P. A network of noncoding regulatory rnas acts in the mammalian brain. _Cell_ 174, 350–362.e17 (2018). Article CAS PubMed PubMed
Central Google Scholar * Bitetti, A. et al. MicroRNA degradation by a conserved target RNA regulates animal behavior. _Nat. Struct. Mol. Biol._ 25, 244–251 (2018). Article CAS PubMed
Google Scholar * Sheu-Gruttadauria, J. et al. Structural basis for target-directed microRNA degradation. _Mol. Cell_ 75, 1243–1255.e7 (2019). Article CAS PubMed PubMed Central Google
Scholar * Bofill-De Ros, X., Yang, A. & Gu, S. IsomiRs: expanding the miRNA repression toolbox beyond the seed. _Biochim. Biophys. Acta Gene Regul. Mech_.
https://doi.org/10.1016/j.bbagrm.2019.03.005 (2019). Article CAS Google Scholar * Yashiro, Y. & Tomita, K. Function and regulation of human terminal uridylyltransferases. _Front.
Genet._ 9, 538 (2018). Article CAS PubMed PubMed Central Google Scholar * Warkocki, Z., Liudkovska, V., Gewartowska, O., Mroczek, S. & Dziembowski, A. Terminal nucleotidyl
transferases (TENTs) in mammalian RNA metabolism. _Philos. Trans. R. Soc. Lond. B. Biol. Sci_. 373, https://doi.org/10.1098/rstb.2018.0162 (2018). Article CAS Google Scholar * D’Ambrogio,
A., Gu, W., Udagawa, T., Mello, C. C. & Richter, J. D. Specific miRNA stabilization by Gld2-catalyzed monoadenylation. _Cell Rep._ 2, 1537–1545 (2012). Article PubMed PubMed Central
CAS Google Scholar * Gutiérrez-Vázquez, C. et al. 3’ Uridylation controls mature microRNA turnover during CD4 T-cell activation. _RNA_ 23, 882–891 (2017). Article PubMed PubMed Central
CAS Google Scholar * Baccarini, A. et al. Kinetic analysis reveals the fate of a microRNA following target regulation in mammalian cells. _Curr. Biol._ 21, 369–376 (2011). Article CAS
PubMed PubMed Central Google Scholar * Tu, B. et al. Distinct and cooperative activities of HESO1 and URT1 nucleotidyl transferases in microRNA turnover in Arabidopsis. _PLoS Genet._ 11,
e1005119 (2015). Article PubMed PubMed Central CAS Google Scholar * Wang, X. et al. Synergistic and independent actions of multiple terminal nucleotidyl transferases in the 3’ tailing
of small RNAs in Arabidopsis. _PLoS Genet._ 11, e1005091 (2015). Article PubMed PubMed Central CAS Google Scholar * Ma, J.-B., Ye, K. & Patel, D. J. Structural basis for
overhang-specific small interfering RNA recognition by the PAZ domain. _Nature_ 429, 318–322 (2004). Article ADS CAS PubMed PubMed Central Google Scholar * Wyman, S. K. et al.
Post-transcriptional generation of miRNA variants by multiple nucleotidyl transferases contributes to miRNA transcriptome complexity. _Genome Res._ 21, 1450–1461 (2011). Article CAS PubMed
PubMed Central Google Scholar * Burroughs, A. M. et al. A comprehensive survey of 3’ animal miRNA modification events and a possible role for 3’ adenylation in modulating miRNA targeting
effectiveness. _Genome Res._ 20, 1398–1410 (2010). Article CAS PubMed PubMed Central Google Scholar * Heo, I. et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through
pre-microRNA uridylation. _Cell_ 138, 696–708 (2009). Article CAS PubMed Google Scholar * Heo, I. et al. Mono-uridylation of pre-microRNA as a key step in the biogenesis of group II
let-7 microRNAs. _Cell_ 151, 521–532 (2012). Article CAS PubMed Google Scholar * Thornton, J. E., Chang, H.-M., Piskounova, E. & Gregory, R. I. Lin28-mediated control of let-7
microRNA expression by alternative TUTases Zcchc11 (TUT4) and Zcchc6 (TUT7). _RNA_ 18, 1875–1885 (2012). Article CAS PubMed PubMed Central Google Scholar * Thornton, J. E. et al.
Selective microRNA uridylation by Zcchc6 (TUT7) and Zcchc11 (TUT4). _Nucleic Acids Res._ 42, 11777–11791 (2014). Article CAS PubMed PubMed Central Google Scholar * Yang, A. et al. 3’
Uridylation confers miRNAs with non-canonical target repertoires. _Mol. Cell_ 75, 511–522.e4 (2019). Article CAS PubMed PubMed Central Google Scholar * Yeom, K.-H. et al.
Single-molecule approach to immunoprecipitated protein complexes: insights into miRNA uridylation. _EMBO Rep._ 12, 690–696 (2011). Article CAS PubMed PubMed Central Google Scholar *
Kim, B. et al. TUT7 controls the fate of precursor microRNAs by using three different uridylation mechanisms. _EMBO J._ 34, 1801–1815 (2015). Article CAS PubMed PubMed Central Google
Scholar * Chang, H.-M., Triboulet, R., Thornton, J. E. & Gregory, R. I. A role for the Perlman syndrome exonuclease Dis3l2 in the Lin28-let-7 pathway. _Nature_ 497, 244–248 (2013).
Article ADS CAS PubMed PubMed Central Google Scholar * Lubas, M. et al. Exonuclease hDIS3L2 specifies an exosome-independent 3’-5’ degradation pathway of human cytoplasmic mRNA. _EMBO
J._ 32, 1855–1868 (2013). Article CAS PubMed PubMed Central Google Scholar * Ustianenko, D. et al. Mammalian DIS3L2 exoribonuclease targets the uridylated precursors of let-7 miRNAs.
_RNA_ 19, 1632–1638 (2013). Article CAS PubMed PubMed Central Google Scholar * Faehnle, C. R., Walleshauser, J. & Joshua-Tor, L. Mechanism of Dis3l2 substrate recognition in the
Lin28-let-7 pathway. _Nature_ 514, 252–256 (2014). Article ADS CAS PubMed PubMed Central Google Scholar * Ghini, F. et al. Endogenous transcripts control miRNA levels and activity in
mammalian cells by target-directed miRNA degradation. _Nat. Commun._ 9, 3119 (2018). Article ADS PubMed PubMed Central CAS Google Scholar * Zhao, Y. et al. The Arabidopsis nucleotidyl
transferase HESO1 uridylates unmethylated small RNAs to trigger their degradation. _Curr. Biol._ 22, 689–694 (2012). Article CAS PubMed PubMed Central Google Scholar * Ren, G., Chen, X.
& Yu, B. Uridylation of miRNAs by hen1 suppressor1 in Arabidopsis. _Curr. Biol._ 22, 695–700 (2012). Article CAS PubMed PubMed Central Google Scholar * Ren, G. et al. Methylation
protects microRNAs from an AGO1-associated activity that uridylates 5’ RNA fragments generated by AGO1 cleavage. _Proc. Natl Acad. Sci. USA_ 111, 6365–6370 (2014). Article ADS CAS PubMed
PubMed Central Google Scholar * Zhai, J. et al. Plant microRNAs display differential 3’ truncation and tailing modifications that are ARGONAUTE1 dependent and conserved across species.
_Plant Cell_ 25, 2417–2428 (2013). Article CAS PubMed PubMed Central Google Scholar * Lim, J. et al. Uridylation by TUT4 and TUT7 marks mRNA for degradation. _Cell_ 159, 1365–1376
(2014). Article CAS PubMed PubMed Central Google Scholar * Liu, X. et al. A MicroRNA precursor surveillance system in quality control of MicroRNA synthesis. _Mol. Cell_ 55, 868–879
(2014). Article CAS PubMed PubMed Central Google Scholar * Reimão-Pinto, M. M. et al. Uridylation of RNA hairpins by tailor confines the emergence of micrornas in drosophila. _Mol.
Cell_ 59, 203–216 (2015). Article PubMed PubMed Central CAS Google Scholar * Bortolamiol-Becet, D. et al. Selective suppression of the splicing-mediated microrna pathway by the terminal
uridyltransferase tailor. _Mol. Cell_ 59, 217–228 (2015). Article CAS PubMed PubMed Central Google Scholar * Pirouz, M., Du, P., Munafò, M. & Gregory, R. I. Dis3l2-mediated decay
is a quality control pathway for noncoding RNAs. _Cell Rep._ 16, 1861–1873 (2016). Article CAS PubMed PubMed Central Google Scholar * Ustianenko, D. et al. TUT-DIS3L2 is a mammalian
surveillance pathway for aberrant structured non-coding RNAs. _EMBO J._ 35, 2179–2191 (2016). Article CAS PubMed PubMed Central Google Scholar * Eckwahl, M. J., Sim, S., Smith, D.,
Telesnitsky, A. & Wolin, S. L. A retrovirus packages nascent host noncoding RNAs from a novel surveillance pathway. _Genes Dev._ 29, 646–657 (2015). Article CAS PubMed PubMed Central
Google Scholar * Reimão-Pinto, M. M. et al. Molecular basis for cytoplasmic RNA surveillance by uridylation-triggered decay in Drosophila. _EMBO J._ 35, 2417–2434 (2016). Article PubMed
PubMed Central CAS Google Scholar * Pisacane, P. & Halic, M. Tailing and degradation of Argonaute-bound small RNAs protect the genome from uncontrolled RNAi. _Nat. Commun._ 8, 15332
(2017). Article ADS CAS PubMed PubMed Central Google Scholar * Pirouz, M., Munafò, M., Ebrahimi, A. G., Choe, J. & Gregory, R. I. Exonuclease requirements for mammalian ribosomal
RNA biogenesis and surveillance. _Nat. Struct. Mol. Biol._ 26, 490–500 (2019). Article CAS PubMed PubMed Central Google Scholar * Jones, M. R. et al. Zcchc11-dependent uridylation of
microRNA directs cytokine expression. _Nat. Cell Biol._ 11, 1157–1163 (2009). Article CAS PubMed PubMed Central Google Scholar * Shukla, S., Bjerke, G. A., Muhlrad, D., Yi, R. &
Parker, R. The RNase PARN controls the levels of specific miRNAs that contribute to p53 regulation. _Mol. Cell_ 73, 1204–1216.e4 (2019). Article CAS PubMed PubMed Central Google Scholar
* Haas, G. et al. Identification of factors involved in target RNA-directed microRNA degradation. _Nucleic Acids Res._ 44, 2873–2887 (2016). Article PubMed PubMed Central Google Scholar
* Hunter, R. W. et al. Loss of Dis3l2 partially phenocopies Perlman syndrome in mice and results in up-regulation of Igf2 in nephron progenitor cells. _Genes Dev._ 32, 903–908 (2018).
Article CAS PubMed PubMed Central Google Scholar * Gu, S., Jin, L., Huang, Y., Zhang, F. & Kay, M. A. Slicing-independent RISC activation requires the argonaute PAZ domain. _Curr.
Biol._ 22, 1536–1542 (2012). Article CAS PubMed PubMed Central Google Scholar * Kim, J. et al. LncRNA OIP5-AS1/cyrano sponges RNA-binding protein HuR. _Nucleic Acids Res._ 44, 2378–2392
(2016). Article CAS PubMed PubMed Central Google Scholar * Belair, C. et al. The RNA exosome nuclease complex regulates human embryonic stem cell differentiation. _J. Cell Biol._ 218,
2564–2582 (2019). Article CAS PubMed PubMed Central Google Scholar * Bofill-De Ros, X. et al. QuagmiR: a cloud-based application for isomiR big data analytics. _Bioinformatics_ 35,
1576–1578 (2019). Article PubMed CAS Google Scholar * Lorenz, R. et al. ViennaRNA Package 2.0. _Algorithms Mol. Biol._ 6, 26 (2011). Article PubMed PubMed Central Google Scholar
Download references ACKNOWLEDGEMENTS We thank Dr. Sandra L. Wolin for critical reading of the manuscript and helpful discussions. This work has been funded by the intramural research program
of the National Cancer Institute, National Institutes of Health (ZIA BC 011566). The Seven Bridges Cancer Genomics Cloud has been funded in whole or in part with Federal funds from the
National Cancer Institute, National Institutes of Health, Contract no. HHSN261201400008C and ID/IQ Agreement no. 17 × 146 under Contract no. HHSN261201500003I. AUTHOR INFORMATION Author
notes * These authors contributed equally: Acong Yang, Tie-Juan Shao, Xavier Bofill-De Ros. AUTHORS AND AFFILIATIONS * RNA Mediated Gene Regulation Section; RNA Biology Laboratory, Center
for Cancer Research, National Cancer Institute, Frederick, MD, 21702, USA Acong Yang, Tie-Juan Shao, Xavier Bofill-De Ros, Chuanjiang Lian, Patricia Villanueva, Lisheng Dai & Shuo Gu *
School of Basic Medicine, Zhejiang Chinese Medical University, Hangzhou, 310053, China Tie-Juan Shao * State Key Laboratory of Veterinary Biotechnology and Heilongjiang Province Key
Laboratory for Laboratory Animal and Comparative Medicine, Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Harbin, 150069, China Chuanjiang Lian Authors *
Acong Yang View author publications You can also search for this author inPubMed Google Scholar * Tie-Juan Shao View author publications You can also search for this author inPubMed Google
Scholar * Xavier Bofill-De Ros View author publications You can also search for this author inPubMed Google Scholar * Chuanjiang Lian View author publications You can also search for this
author inPubMed Google Scholar * Patricia Villanueva View author publications You can also search for this author inPubMed Google Scholar * Lisheng Dai View author publications You can also
search for this author inPubMed Google Scholar * Shuo Gu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.Y., T.J.S., X.B.-D.R., and S.G.
designed the research. A.Y. and T.J.S. performed the most experiments with helps from C.L., P.V., and L.D.; X.B.-D.R. did all the bioinformatic analyses; A.Y., T.J.S., X.B.-D.R., and S.G.
analyzed the data. A.Y., T.J.S., X.B.-D.R., and S.G. wrote the paper. CORRESPONDING AUTHOR Correspondence to Shuo Gu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks Bin Yu, Jia Yu and the other, anonymous, reviewer(s) for their contribution to the peer review of
this work. Peer reviewer reports are available. PUBLISHER′S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative
Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the
original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in
the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended
use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yang, A., Shao, TJ., Bofill-De Ros, X. _et al._ AGO-bound mature miRNAs are
oligouridylated by TUTs and subsequently degraded by DIS3L2. _Nat Commun_ 11, 2765 (2020). https://doi.org/10.1038/s41467-020-16533-w Download citation * Received: 27 October 2019 *
Accepted: 30 April 2020 * Published: 02 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16533-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative