Play all audios:
ABSTRACT NF-Kappa B has a significant role in inflammatory processes as well as in colorectal cancer. The aim of this study was to compare the expression of NF-kappa B in colonic
adenocarcinoma specimen, colonic adenomas and inflammatory colonic tissues. Patients with colorectal cancer (CRC), colonic adenomas and inflammatory processes undergoing surgery were
recruited. Following a routine pathological evaluation tissue samples were stained using anti NF-κB monoclonal antibodies. Expression of NF-κB was quantified using IMAGEJ program for
immunohistochemistry staining. Samples were also stained and quantified for CEA expression. Fifty-six patients were included. 30 cancers, 6 polyps and 20 inflammatory processes. Expression
of NF-κB was similar between polypoid and inflammation etiologies. However, it was significantly higher in CRC compared to both (_p_ < 0.05). In cancer patients, NF-κB expression in the
resection margins was correlated with positive node status. CEA expression was higher in the cancer group, less in the IBD group and the lowest in the colonic non diseased margins. Our
results provide a supportive evidence that NF-κB pathway is strongly involved in colon cancer development and metastasis. Interestingly, expression of NF-κB in benign polypoid lesions was as
high as in inflammatory etiologies. This support the role of NF-κB early in the adenoma to carcinoma sequence. Further research is needed to evaluate the exact role of NF-κB in tumor
progression in order to look for diagnostic and therapeutic possibilities. SIMILAR CONTENT BEING VIEWED BY OTHERS LOSS OF SATB2 EXPRESSION CORRELATES WITH CYTOKERATIN 7 AND PD-L1 TUMOR CELL
POSITIVITY AND AGGRESSIVENESS IN COLORECTAL CANCER Article Open access 09 November 2022 JAML OVEREXPRESSED IN COLORECTAL CANCER PROMOTES TUMOUR PROLIFERATION BY ACTIVATING THE PI3K-AKT-MTOR
SIGNALLING PATHWAY Article Open access 18 October 2024 CYTOKERATIN 7 EXPRESSION AS A PREDICTOR OF AN UNFAVORABLE PROGNOSIS IN COLORECTAL CARCINOMA Article Open access 09 September 2021
INTRODUCTION The link between inflammation and tumorigenesis is well-established. It was first described in epidemiological studies indicating that chronic inflammation predisposes
individuals to different cancers, including Colorectal Cancer (CRC)1,2. This was followed by evidence of how non-steroidal anti-inflammatory drugs (NSAIDs) decrease the prevalence of several
tumors [reviewed in 1]. Finally, numerous mechanistic links between the two began to emerge, including NF-κB, which is discussed here1,3. This association has been demonstrated in several
tissue types and organs, including the colon1. CRC is one of the most common types of cancer in western countries. Sporadic, as well as colitis-associated CRC, develop gradually and present
treatment challenges and greater likelihood of mortalityin advanced stages4. Much research has focused on characterizing new markers for early detection and staging of this type of cancer2.
In parallel, in the context of high-risk IBD patients, a novel follow-up tool for early detection of the inflammation to cancer transition would have high clinical value. NF-κB is an
intracellular transcription factor with a distinct role in several processes related to inflammation and cancer3. NF-κB can become activated in response to extracellular stimuli, such as
cytokines, growth factors, oncoproteins and stress signals5. Tumor necrosis factor alpha (TNFα) is the most well-known factor that triggers the NF-κB signaling pathway via its receptor.
Other activating factors include interleukin-1 and toll-like receptor ligands. Following activation, the NF-κB intracellular cascade can act both in the cytoplasm and in the nucleus to
stimulate family proteins and target genes, thereby generating its diverse effects6. In cellular models for colon cancer, induction of NF-κB was shown to promote tumor growth by upregulating
and cross-talking with key signaling pathways, such as the phosphoinositide 3-kinase (PI3K)/Akt cascade, the cell-cycle process and anti-apoptotic pathways. NF-κB is also able to prompt
production of proangiogenic and fundamental invasiveness factors, including cyclooxygenase-2 (COX2), growth factors, interleukins, cell adhesion molecules and matrix metalloproteinases6,7.
Involvement of NF-κB in all these pathways has a major effect on immune and inflammatory processes8. Namely, the NF-κB pathway is involved in the development of primary lymphoid organs and
in the early formation and subsequent long-term maintenance of immune tolerance. It enables antigen-presenting cells of the innate immunity to communicate with the adaptive immunity, which
is required for the survival and maturation of B cells in peripheral lymphoid organs (i.e., humoral immunity) and for regulating T cell responses (i.e., adaptive immunity). Since NF-κB has a
substantial role in inflammatory processes and in cancer development, this study aimed to evaluate NF-κB expression in colonic tissue of patients with colorectal cancer, IBD and colonic
polyps. A comparison of NF-κB expression between the three enteritis was performed by quantifying the immunohistochemically staining. In parallel we have looked also on CEA expression in
these tissues as CEA is known to be overexpressed also in both IBD9 and in colorectal cancer10. METHODS This prospective, non-interventional study was approved by the Meir Medical Center
Institutional Review Board, and was performed in accordance with the ethical standards laid down in the Declaration of Helsinki. All patients provided written informed consent before being
included in this study. Patients with malignant or benign colorectal diseases who were referred for elective surgery at Meir Medical Center from 2012 to 2018 were consecutively recruited to
enroll in this non-interventional cohort study. AJCC staging system was used to characterize CRC patients11. Patients with distant metastasis discovered before or during surgery and those
with positive macroscopic or microscopic resection margins were excluded. IMMUNOHISTOCHEMICALLY STAINING FOR NF-ΚB The colorectal resection specimens were submitted for histopathological
examination, according to routine protocol. All tissue samples were reviewed for each case. Two paraffin blocks were selected for the project: (a) pathological tissue from the primary tumor
or the site of the benign pathology and (b) and from surgical margins. Four 4 µm-thick sections were cut from selected tissue blocks, embedded in xylene and dehydrated. They were further
processed for NF-κB immunohistochemistry staining, according to the manufacturer’s protocol (MERCK Anti-NF-κB, P65, clone E379. Monoclonal Antibody. Millipore™) with an antibody dilution of
1:50,000. The immunostains were carried out on using Ventana BenchMark Ultra Automated Stainer (Roche Diagnostics, Basel, Switzerland). As a positive control for NFkB staining we used
mammary lymph nodes and for negative control primary antibodies were excluded from the staining procedure. all in accordance with the manufacturer's instructions and the supervision of
our Pathology immunohistochemistry specialist. QUANTIFICATION OF TISSUE NF-ΚB LEVELS Digital images were captured from the stained slides using the Olympus BX41 microscope, equipped with an
Olympus DP73 camera and were 4 times magnified . Captures of four different fields were taken from every slide (× 4, 381*513 sq-pixels) and saved using Olympus Entry cellSens software12.
NF-κB tissue quantification levels were calculated using IMAGEJ program for IHC, using the ImageJ Colour Deconvolution filter to distinguish NF-κB staining from hematoxylin and separate DAB
staining13,14. Data values were normalized per area and then converted to optical density (OD) measurements using the Rodbar function15: $${\text{OD}} = \log {\text{
}}({\text{max}}\,{\text{intensity}}/{\text{mean}}\,{\text{intensity}}),{\text{ where}}\,{\text{max}}\,{\text{intensity }} = {\text{ }}255\,{\text{for}}\,8 - {\text{bit}}\,{\text{images}}.$$
Captured fields and/or missing tissue may deviate and cofound calculations. Thus, we selected only fields containing tissue either with no empty areas or those as small as possible.
STATISTICS Statistical analysis was performed using Statistical Package for Social Sciences, Version 25.0 (IBM, Armonk, NY, USA). The data are described as numbers and percentages for
nominal parameters and as means and standard deviations for continuous variables. Differences between characteristic qualitative variables and NF-κB levels were compared using t-test and
Mann–Whitney U test. Mean optical density, standard deviation and p-values in Mann–Whitney correlations are shown in the tables and figures. Correlations between two continuous variables
were evaluated with Pearson's correlation and Mann–Whitney test. Chi-square test was used to analyze categorical variables. P-values less than 0.05 were considered statistically
significant. RESULTS A total of 56 patients were included in this study, 30 with a CRC diagnosis and 26 with benign conditions of the colon (Table 1). NF-κB levels in the pathological tissue
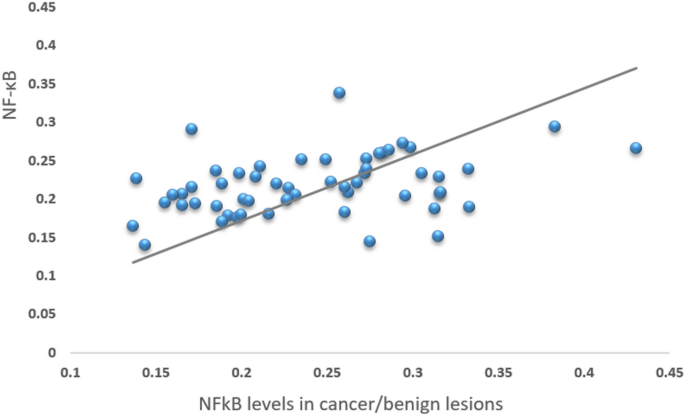
positively correlated with levels in tissue margins in all 56 patients (Fig. 1). NF-κB expression in the cancerous tissues was significantly elevated compared to NF-κB expression in tissues
of patients with adenomatous polyposis, to its expression in the epicenter of patients with all kinds of inflammatory processes and to its expression in the epicenter of the inflammation
process in IBD patients (Fig. 2). Expression of NF-Κb was higher in the cancerous tissues compared to the normal margins of resection in the same patients (Fig. 3). Interestingly expression
of NF-Κb in the inflammation epicenter of patients with IBD was similar to its expression in the resection margins of these patients. However, in 9 out of the 16 patients with IBD (56%) the
resection margins had signs of inflammation in histology. Histological images of cancer and Crohn’s disease are presented in Fig. 4. A comparison of NF-κB levels between tissues of CRC
patients with or without positive lymph nodes revealed elevated expression of NF-κB in the margins of CRC patients’ specimen that had positive nodes. Expression of NF-κB in the tumoral
tissues was also slightly higher in the nodes’ positive group, however, it did not reach statistically significance (Table 2). NF-κB tumor expression did not correlate with the level of
local tumor invasiveness (T stage). Long term follow-up revealed four patients out of the 30 cancer patients that have developed disease recurrence. The mean ± SD of NF-kB expression was
compared between these 4 patients and the others (Fig. 5). A Mann–Whitney correlation test did not reveal a statistical significant difference between the two groups (p = 0.58). A comparison
of CEA expression between cancer tissues, IBD tissues and resection margins of CRC (defined here as the control normal tissue) demonstrated high CEA expression in the cancer group, less in
the IBD group and lowest in the colonic non diseased margins (Fig. 6). A Pearson correlation test did not reveal a statistical significant correlation between CEA and NFKB expression in
cancer patients (Table 3). DISCUSSION There is growing evidence linking inflammation, cancer development, and nuclear factor kappa B (NF-κB)1. Preclinical studies using animal models have
shown that NF-κB is involved in colorectal carcinogenesis. In mice models, the NF-κB pathway has been directly linked to intestinal inflammation and to development of colitis-associated
cancer16,17,18. In mice models of CRC, NF-κB has also been linked to progression of tumor growth17,19. Yet, the clinical implications of NF-κB in human inflammatory or cancer diseases of the
colon have rarely been studied and evidence for its relevance to these patients is limited20. To date, activating mutations of NF-κB in CRC have not been reported3. Nonetheless,
constitutive activation of NF-κB has been observed in human CRC and associated with higher tumor stage18,21, treatment resistance19,22 and poor survival outcomes3. In addition, activation of
the NF-κB main protein p65 in colon of metastatic CRC patients correlated with liver metastasis and poor clinical outcomes, measured as overall survival; suggesting it may have prognostic
value in this disease17,23. In this study, we evaluated the levels of RelA/p65 protein, which is a key in the canonical pathway of NF-κB activated by tumor necrosis factor alpha, Toll-like
receptor ligands, and interleukin-15. RelA/p65 is less involved in the non-canonical pathway, activated by other ligands and signals. Via the canonical pathway, different combinations of
subunits with RelA/p65 protein are shown to activate this signaling pathway, regardless of the specific dimer formed5,6. RelA/p65 protein was chosen based on the intent to compare protein
expression between inflammatory conditions of the colon and those of cancerous conditions. In other words, since this is a basic proof of concept study we went for a gross evaluation and
looked for the wide picture. Nonetheless, here we evaluated the expression levels of RelA/p65 NF-κB protein in colonic tissues of patients undergoing surgery for various etiologies. We have
found that NF-κB levels were significantly higher in CRC cancer compared to tumor-free margins and to benign tissues, including IBD specimens. We consider this novel finding to be
unpredicted since the role of the NF-κB signaling in inflammation is well-established compared to its involvement in CRC. Thus, we did not anticipate NF-κB levels to be significantly higher
in CRC compared to tumor-free IBD specimens. Our findings stressed the important role of NF-κB in colorectal cancer. Additionally, we have found that the expression of NF-κB in adenomatous
polyps was similar to its expression in IBD tissues. This finding may support the hypothesis that NF-κB plays an important role early in the process of colonic dysplasia development that may
lead to cancer. Interestingly, this finding correlates to other studies linking between NF-κB and colitis-associated adenoma development24. Some studies tried to look for an association of
NF-κB to cancer aggressiveness. Pyo et. al. investigated the correlation between phosphorylated NF-κB (pNF-κB, the activated form of the NF-κB protein) nuclear expression in pathological
tissues and clinicopathological characteristics of CRC patients20. That study included 261 patients. They have demonstrated that pNF-κB was significantly correlated with frequent perineural
invasion, lymph node metastasis and higher disease stage. The relation to disease stage was attributed to the association with positive lymph nodes but not to the tumor T stage, which was
not correlated with pNF-κB levels. These findings are comparable in some aspects to our findings. Interestingly, in our study, NF-κB expression in the margins of resection was associated
with positive node status of CRC patients. This may lead to the assumption that the associated processes that link NF-κB with colorectal cancer is related to the entire colonic tissue and
not only to the tumor site itself. In our study, we could not show a statistical significance levels of NF-κB expression in the tumoral tissue itself between negative and positive nodes
status, however, this may be related to the low number of patients we had in these groups. Nevertheless, based on our results and others20, this indicates that NF-κB signaling may be
involved in the dissemination processes of colonic adenocarcinoma cells. But, as its levels have not correlated with the extent of tumor involvement (T stage), we believe that NF-κB is more
involved in the tumor metastatic process rather than direct invasiveness. This data supports the hypothesis on the role of NF-κB in colorectal carcinogenesis, underlining the point that this
signaling pathway may be more significant when involved in colon cancer dissemination mechanisms than in the primary colonic carcinogenic process. In our study 4 out of 30 patients have
developed recurrence. We could not show a statistical significant difference in the expression NF-κB when comparing these patients with patients that had no recurrence despite a numerical
difference (Fig. 5). We believe that this may be related to the low number of patients in our series that does not allow a reliable statistical evaluation. To further assess this hypothesis,
we suggest a long-term, prospective clinical study measuring NF-κB expression levels of CRC patient colonic specimens matched with long-term follow-up data regarding metastatic disease
recurrence. Furthermore, our data revealed that NF-κB tumor levels positively correlated with expression in tissue margins (Fig. 1), which demonstrates that the activation of this signaling
pathway in colons with pathological tissue is diffuse and not limited to the tumor mass. This interesting finding may suggest either a predisposition to increased NF-κB expression among
patients with colonic pathologies or a remote effect of the primary lesion on the adjacent normal colonic mucosa. This also supports the concept that NF-κB may affect the metastatic
potential of the tumor to its surrounding tissue more broadly than the tumor itself does. As activation of NF-κB pathways has been previously associated with poor prognosis, it is possible
that members of the NF-κB pathways could serve as prognostic markers or novel therapeutic targets for CRC. Indeed, several suggestions for anti-NF-κB therapeutics for colon cancer treatment
have been examined in pre-clinical trials [reviewed in 5,25]. But despite the promising results, no clinical trials have been published to date. Thus, there is a lack of clinical evidence to
validate this potential. Another point regarding the association of NF-κB with poor prognosis is its role in cancer resistance to therapy. NF-κB activation has been suggested to be
associated with resistance to therapy in gastrointestinal malignancies, mainly in predicting resistance to chemoradiation in esophageal cancer26 and to a combination of irinotecan and
cetuximab in CRC27. Neither of these studies, however, addressed the possibility that NF-κB is a prognostic rather than a predictive clinical marker. Furthermore, the literature on the role
of NF-κB in CRC progression is limited [reviewed in 5 and 25]. One of the limitation of our study is the lack of evaluation of other important biomarkers that are known to activate or be
activated by NF-κB. Two reviews on the association between the inflammation process of IBD and the development of colorectal cancer have stressed the relation between certain cytokines such
as IL-6, IL- α and TNF-α to NF-κB and proposed that its mechanism of action is related to its ability to block apoptosis by positively regulating the expression of anti-apoptosis
proteins28,29. Our study did not aim to explore the mechanism of action of NF-κB but to demonstrate another evidence from a different angle for its pivotal role in the development of
colorectal cancer. Our study is novel in its methods (quantifying the immunochemistry staining in a relatively new method) and in its idea (comparing expression of NF-κB between cancer and
IBD). Adding a western blot and Qpcr could have further verifying the expression of NF-KB, however, the technique that we have used to quantify NF-KB is a well-established and reliable
one13,14. The results further support the accumulating data on the important role of NF-κB in CRC and should encourage other researchers to use this method and to further investigate the
expression of NF-κB in IBD patients with and without cancer. We have added in our study another evaluation of a biomarker, CEA. CEA is not directly related to NF-Κb and thus We did not
expect to find a correlation between CEA and NF-KB. however, CEA is known to be highly associated with both CRC and IBD. Thus, the difference that we have found between CEA expression in
cancer tissue compared to IBD that goes along with the difference in NF-κB expression supports the validity of our methods and findings. CONCLUSIONS The results of this study add clinical
evidence to the physiological involvement of NF-κB in the progression and dissemination of colon cancer. Confined with the existing literature describing the role of NF-κB in inflammation
and cancer, these findings support the role of the NF-κB signaling pathway in CRC development, its potential role in tumor metastasis and its potential as a target for therapy. Additional
clinical research is needed to further validate the relevance of NF-κB tumor expression to patient prognosis in terms of recurrence and long-term survival, and clarify its role in
inflammation during tumorigenesis, with or without the presence of IBD. DATA AVAILABILITY The data that support the findings of this study are available on request from the corresponding
author. The data are not publicly available due to privacy or ethical restrictions. REFERENCES * Wang, S., Liu, Z., Wang, L. & Zhang, X. NF-kappaB signaling pathway, inflammation and
colorectal cancer. _Cell Mol. Immunol._ 6, 327–334. https://doi.org/10.1038/cmi.2009.43 (2009). Article CAS PubMed PubMed Central Google Scholar * Rizzo, L., Pallone, F., Monteleone, G.
& Fantini, M. C. Intestinal inflammation and colorectal cancer: a double-edged sword?. _World J. Gastroenterol._ 17(26), 3092–3100. https://doi.org/10.3748/wjg.v17.i26.3092 (2011).
Article PubMed PubMed Central Google Scholar * Ben-Neriah, Y. & Karin, M. Inflammation meets cancer, with NF-κB as the matchmaker. _Nat. Immunol._ 12, 715–723.
https://doi.org/10.1038/ni.2060 (2011). Article CAS PubMed Google Scholar * Simon, K. Colorectal cancer development and advances in screening. _Clin. Interv. Aging._ 19(11), 967–976.
https://doi.org/10.2147/CIA.S109285 (2016). Article Google Scholar * Patel, M., Horgan, P. G., McMillan, D. C. & Edwards, J. NF-κB pathways in the development and progression of
colorectal cancer. _Transl. Res._ 197, 43–56. https://doi.org/10.1016/j.trsl.2018.02.002 (2018). Article CAS PubMed Google Scholar * Vaiopoulos, A. G., Athanasoula, KCh. &
Papavassiliou, A. G. NF-κB in colorectal cancer. _J. Mol. Med. (Berl)._ 91(9), 1029–1037. https://doi.org/10.1007/s00109-013-1045-x (2013). Article CAS PubMed Google Scholar *
Vaiopoulos, A. G., Papachroni, K. K. & Papavassiliou, A. G. Colon carcinogenesis: learning from NF-kappaB and AP-1. _Int. J. Biochem. Cell Biol._ 42(7), 1061–1065.
https://doi.org/10.1016/j.biocel.2010.03.018 (2010). Article CAS PubMed Google Scholar * Sun, S. C. The non-canonical NF-κB pathway in immunity and inflammation. _Nat. Rev. Immunol._
17(9), 545–558. https://doi.org/10.1038/nri.2017.52 (2017). Article CAS PubMed PubMed Central Google Scholar * Kelleher, M., Singh, R., O’Driscoll, C. M. & Melgar, S.
Carcinoembryonic antigen (CEACAM) family members and inflammatory bowel disease. _Cytokine Growth Factor Rev._ 47, 21–31 (2019). Article CAS Google Scholar * Li, M. _et al._ Comparison of
carcinoembryonic antigen prognostic value in serum and tumour tissue of patients with colorectal cancer. _Colorectal Dis._ 11(3), 276–281 (2009). Article CAS Google Scholar * Edge, S. B.
& Compton, C. C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. _Ann. Surg. Oncol._ 17(6), 1471–1474 (2010). Article
Google Scholar * Kuhn, E., Ayhan, A., Shih, I. M., Seidman, J. D. & Kurman, R. J. Ovarian Brenner tumour: A morphologic and immunohistochemical analysis suggesting an origin from
fallopian tube epithelium. _Eur. J. Cancer_ 49(18), 3839–3849 (2013). Article Google Scholar * Rong, Fu., Ma, X., Bian, Z. & Ma, J. Digital separation of diaminobenzidine-stained
tissues via an automatic color-filtering for immunohistochemical quantification. _Biomed. Opt. Exp._ 6(2), 544–558. https://doi.org/10.1364/BOE.6.000544 (2015). Article CAS Google Scholar
* Varghese, F., Bukhari, A. B., Malhotra, R. & De, A. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human
tissue samples. _PLoS One._ 9(5), e96801. https://doi.org/10.1371/journal.pone.0096801 (2014). Article ADS CAS PubMed PubMed Central Google Scholar * Joers, V. _et al._ Cardiac
sympathetic denervation in 6-OHDA-treated nonhuman primates. _PLoS ONE_ 9(8), e104850. https://doi.org/10.1371/journal.pone.0104850 (2014). Article ADS PubMed PubMed Central Google
Scholar * Greten, F. R. _et al._ IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. _Cell_ 118, 285–296. https://doi.org/10.1016/j.cell.2004.07.013
(2004). Article CAS PubMed Google Scholar * Klampfer, L. Cytokines, inflammation and colon cancer. _Curr. Cancer Drug Targets._ 11, 451–464. https://doi.org/10.2174/156800911795538066
(2011). Article CAS PubMed PubMed Central Google Scholar * Terzic, J., Grivennikov, S., Karin, E. & Karin, M. Inflammation and colon cancer. _Gastroenterology_ 138, 2101–2114.
https://doi.org/10.1053/j.gastro.2010.01.058 (2010). Article CAS PubMed Google Scholar * Vlantis, K. _et al._ Pasparakis M Constitutive IKK2 activation in intestinal epithelial cells
induces intestinal tumors in mice. _J. Clin. Invest._ 121, 2781–2793 (2011). Article CAS Google Scholar * Pyo, J. S. & Kim, E. K. Clinicopathological significance and prognostic
implication of nuclear factor-κB activation in colorectal cancer. _Pathol. Res. Pract._ 215(8), 152469. https://doi.org/10.1016/j.prp.2019.152469 (2019). Article CAS PubMed Google Scholar
* Kojima, M. _et al._ Increased nuclear factor-kB activation in human colorectal carcinoma and its correlation with tumor progression. _Anticancer Res._ 24(2), 675–81 (2004). CAS PubMed
Google Scholar * Lind, D. S. _et al._ Nuclear factor-κB is upregulated in colorectal cancer. _Surgery_ 130, 363–369. https://doi.org/10.1067/msy.2001.116672 (2001). Article CAS PubMed
Google Scholar * Puvvada, S. D. _et al._ NF-kB and Bcl-3 activation are prognostic in metastatic colorectal cancer. _Oncology_ 78(3–4), 181–188. https://doi.org/10.1159/000313697 (2010).
Article CAS PubMed PubMed Central Google Scholar * Burkitt, M. D. _et al._ NF-κB1, NF-κB2 and c-Rel differentially regulate susceptibility to colitis-associated adenoma development in
C57BL/6 mice. _J. Pathol._ 236(3), 326–36 (2015). Article CAS Google Scholar * Hassanzadeh, P. Colorectal cancer and NF-κB signaling pathway. _Gastroenterol. Hepatol. Bed. Bench._ 4(3),
127–132 (2011). PubMed PubMed Central Google Scholar * Izzo, J. G. _et al._ Association of activated transcription factor nuclear factor kappab with chemoradiation resistance and poor
outcome in esophageal carcinoma. _J. Clin. Oncol._ 24, 748–754. https://doi.org/10.1200/JCO.2005.03.8810 (2006). Article CAS PubMed Google Scholar * Scartozzi, M. _et al._ Nuclear
factor-κB tumor expression predicts response and survival in irinotecan-refractory metastatic colorectal cancer treated with cetuximab-irinotecan therapy. _J. Clin. Oncol._ 25, 3930–3935.
https://doi.org/10.1200/JCO.2007.11.5022 (2007). Article CAS PubMed Google Scholar * Viennois, E., Chen, F. & Merlin, D. NF-κB pathway in colitis-associated cancers. _Transl.
Gastrointest. Cancer._ 2(1), 21–29 (2013 Jan 1). CAS PubMed PubMed Central Google Scholar * Romano, M. _et al._ From inflammation to cancer in inflammatory bowel disease: molecular
perspectives. _Anticancer Res._ 36(4), 1447–1460 (2016). CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS The authors thank Nava Jelin for the statistical analysis and Faye
Schreiber for language editing. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Surgery B and Cancer Research Lab, Meir Medical Center, Kfar Saba, Israel Liron Berkovich, Mirit
Gerber & Shmuel Avital * The Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel Liron Berkovich, Mirit Gerber & Shmuel Avital * Department of Pathology, Meir Medical
Center, Kfar Saba, Israel Aviva Katzav & Debora Kidron Authors * Liron Berkovich View author publications You can also search for this author inPubMed Google Scholar * Mirit Gerber View
author publications You can also search for this author inPubMed Google Scholar * Aviva Katzav View author publications You can also search for this author inPubMed Google Scholar * Debora
Kidron View author publications You can also search for this author inPubMed Google Scholar * Shmuel Avital View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS M.G., L.B., A.K. and D.K. did the actual laboratory research including all the different staining of the histological slides. L.B. did the quantification of the tissue
staining . I.B. prepared all figures. S.A. led the group and together with liron berkovitch wrote the main mqnuscript. All authors reviewed and aproved the final version of the manuscript.
CORRESPONDING AUTHOR Correspondence to Shmuel Avital. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are
included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons
licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of
this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Berkovich, L., Gerber, M., Katzav, A. _et al._ NF-kappa B
expression in resected specimen of colonic cancer is higher compared to its expression in inflammatory bowel diseases and polyps. _Sci Rep_ 12, 16645 (2022).
https://doi.org/10.1038/s41598-022-21078-7 Download citation * Received: 11 June 2022 * Accepted: 22 September 2022 * Published: 05 October 2022 * DOI:
https://doi.org/10.1038/s41598-022-21078-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative