Play all audios:
ABSTRACT The right ventricular (RV) impairment can predict clinical adverse events in patients following transcatheter aortic valve replacement (TAVR) for severe aortic stenosis (AS).
Limited reports have compared impact of the left ventricular (LV) and RV disorders. This retrospective study evaluated two-year major adverse cardiac and cerebrovascular events (MACCE) in
patients following TAVR for severe AS. RV sphericity index was calculated as the ratio between RV mid-ventricular and longitudinal diameters during the end-diastolic phase. Of 239 patients,
2-year MACCE were observed in 34 (14%). LV ejection fraction was 58 ± 11%. Tricuspid annular plane systolic excursion (TAPSE) and RV sphericity index were 20 ± 3 mm and 0.36 (0.31–0.39).
Although the univariate Cox regression analysis demonstrated that both LV and RV parameters predicted the outcomes, LV parameters no longer predicted them after adjustment. Lower TAPSE
(adjusted hazard ratio per 1 mm, 0.84; 95% confidence interval, 0.75–0.93) and higher RV sphericity index (adjusted hazard ratio per 0.1, 1.94; 95% confidence interval, 1.17–3.22) were
adverse clinical predictors. In conclusion, the RV structural and functional disorders predict two-year MACCE, whereas the LV parameters do not. Impact of LV impairment can be attenuated
after development of RV disorders. SIMILAR CONTENT BEING VIEWED BY OTHERS LONG-TERM SURVIVAL EVALUATION AFTER TRANSCATHETER AORTIC VALVE IMPLANTATION IN PATIENTS WITH SEVERE AORTIC VALVE
STENOSIS: A RETROSPECTIVE COHORT STUDY Article Open access 01 April 2025 CLASSIFICATION OF SEVERE AORTIC STENOSIS AND OUTCOMES AFTER AORTIC VALVE REPLACEMENT Article Open access 07 May 2022
NOVEL ECHOCARDIOGRAPHY-DERIVED LEFT VENTRICULAR STIFFNESS INDEX IN LOW-FLOW VERSUS NORMAL-FLOW SEVERE AORTIC STENOSIS WITH PRESERVED LEFT VENTRICULAR EJECTION FRACTION Article Open access 03
June 2020 INTRODUCTION Transcatherter aortic valve replacement (TAVR) has been emerged as alternative therapy for patients with severe aortic stenosis (AS) at high or prohibitive surgical
risk1 and can provide better prognosis in such population. However, even after the procedure, major adverse cardiac and cerebrovascular events (MACCE) such as heart failure hospitalization
may occur and result in impaired activity daily living, higher cost, higher incidences of mortality and morbidities2. It would be necessary to identify patients at a risk of clinical adverse
events after TAVR for suppression of cost and improvement in prognosis. According to current guidelines, surgical or transcatheter aortic valve replacement (AVR) is determined based on the
demonstration of severe AS, presence or absence of symptoms related to AS, and left ventricular ejection fraction (LVEF)3. The AVR decision algorithm does not include cardiac structural and
functional findings other than LVEF. Of cardiac impairment, the right heart structure and function, which have been largely underestimated in the left heart diseases4, have attracted
attention of clinicians and researchers for the last few years. Indeed, both structural and functional impairment of the right heart predicted worse clinical outcomes in patients who
underwent TAVR according to some recent studies5,6,7,8. The conception of staging classification based on the extra-aortic valve cardiac damage supports the importance of the right heart
findings9 and may suggest that the right ventricular (RV) dysfunction reflects the advanced left ventricular (LV) dysfunction10. It should be clarified which, or both of the LV and RV
characteristics is important to predict adverse clinical events. Understanding of any interactions between the LV and RV (or the left and right heart system) disorders may contribute to
better patient selection for TAVR. The present study aimed to (1) identify predictors related to the RV structure and function, (2) demonstrate whether the RV dysfunction may predict adverse
clinical outcomes more accurately than that of the LV, and (3) investigate which LV parameters are associated with the RV findings in patients who underwent TAVR for severe AS. METHODS
STUDY POPULATION The current study was conducted based on a single-center, retrospective registry. Treatment option, surgical aortic valve replacement or TAVR, was determined by an
interdisciplinary heart team at our hospital, considering the severity of AS, echocardiographic findings, symptoms, comorbidities including frailty, and life expectancy. TAVR was selected
for patients at high surgical risk or those who were not considered to be suitable candidates for surgery because they had coexisting conditions11. INCLUSION AND EXCLUSION CRITERIA
Consecutive patients following TAVR at our hospital between 2015 and 2022 were included. Patients who underwent TAVR in surgical aortic valve (SAV) for aortic regurgitation (AR) were
excluded from the present analysis. DATA COLLECTION Patient characteristics including age, sex, comorbidities, medical history, laboratory data, echocardiographic findings, oral medication
at discharge, and 2-year cardiovascular events were collected. The laboratory and echocardiographic data were obtained from an electronic medical record at the nearest day within the last
month prior to TAVR. Sapien XT, Sapien 3 (Edwards Lifesciences, Irvine, CA, USA), Evolut R, Evolut Pro, or Evolut Pro Plus (Medtronic, Minneapolis, MN, USA) was selected for the procedure.
The transfemoral, transapical, or transsubclavian/transaxillary approach was applied. STUDY ENDPOINT This study assessed 2-year MACCE, a composite of cardiovascular death, hospitalization
for heart failure, nonfatal acute myocardial infarction, and cerebral stroke. The follow-up was completed on the last medical interview date, last examination date, or date when an endpoint
event was observed, whichever came first. Patients who become lost to follow-up at 2 years were censored. All data concerning the follow-up duration and adverse clinical events at 2 years
were acquired from medical records or telephone interview. MACCE was confirmed by dedicated cardiologists that adjudicates all clinical events. ECHOCARDIOGRAPHIC FINDINGS Echocardiography
was conducted and analyzed by experienced cardiologists or clinical technologists. All patients underwent transthoracic echocardiography prior to TAVR. Severity of AS was determined based on
the max velocity, mean pressure gradient, or AV area according to the ACC/AHA guideline3. LVEF, left ventricular end-diastolic volume (LVEDV), and left ventricular end-systolic volume
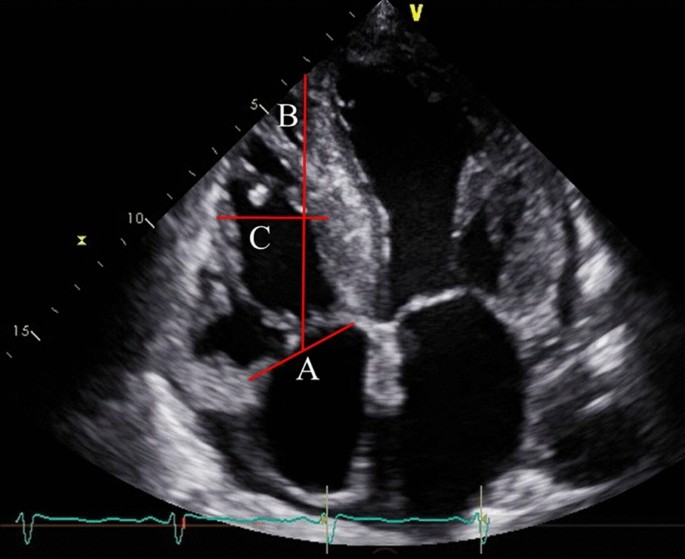
(LVESV) were assessed by using modified Simpson’s biplane method. The right ventricular (RV) and atrial size and the diameter of the inferior vena cava were measured. RV sphericity index was
calculated as the ratio between RV mid-ventricular and longitudinal diameters during the end-diastolic phase (Fig. 1)12, which is an indicator of RV remodeling13. LV sphericity index was
also determined using same method as the RV sphericity index. Tricuspid annulus diameter was also measured during the end-diastolic phase. Systolic right ventricular function was assessed by
TAPSE and RV fractional area change. Systolic pulmonary artery pressure (SPAP) was determined by measurement of the maximal tricuspid regurgitation velocity–derived gradient by continuous
wave Doppler, inferior vena cava diameter, and respiratory-related changes in inferior vena cava diameter14. RV-PA coupling, which represents the association between the right ventricular
contractility and pulmonary afterload, was defined as TAPSE-to-SPAP ratio (mm/mm Hg). Severity of mitral regurgitation (MR) and tricuspid regurgitation (TR) was scored on a scale ranging
from 1 + (mild) to 4 + (severe)15 and 1 + (mild) to 5 + (torrential)16, respectively. None or trivial regurgitation was categorized as 0. MR and TR vena contracta (VC) were evaluated during
the mid-systolic phase. DEFINITIONS Reduced LVEF was defined as LVEF less than 40%. Pulmonary hypertension (PH) was determined if SPAP was 38 mmHg or higher according to transthoracic
echocardiography17. Chronic kidney disease (CKD) stages 3B–5 was diagnosed based on an estimated glomerular filtration rate < 45 ml/min/1.73 m218. Baseline laboratory evaluation of
hepatobiliary function included total bilirubin (TB), alkaline phosphatase (ALP), and GGT. TB was considered abnormal if it exceeded 1.2 mg/dL, irrespective of sex. For ALP and GGT, we used
sex-specific laboratory cutoff values as follows: ALP, 130 U/L (males) and 105 U/L (females); gamma glutamyl transferase (GGT), 59 U/L (males) and 39 U/L (females). Hepatobiliary system
impairment was defined as elevation of at least two of three parameters (TB, ALP, and GGT)19. Patients were classified based on aortic mean pressure gradient (dPmean), stroke volume index
(SVi), and LVEF into four groups: (1) dPmean of ≥ 40 mmHg (high-gradient [HG]); (2) dPmean of < 40 mmHg and LVEF of < 50% (classical low-flow low-gradient [LFLG]); (3) dPmean of <
40 mmHg, LVEF of ≥ 50% and SVi of ≤ 35 ml/m2 (paradoxical LFLG)20; (4) dPmean of < 40 mmHg, LVEF of ≥ 50%, and SVi of > 35 mi/m2 (normal-flow low-gradient [NFLG]). Patients with
classical LFLG underwent dobutamine stress echocardiography. The Clinical Frailty Scale was assessed as a marker of physical activity. This scale ranges 1 (very fit) to 9 (terminal ill),
with a higher number indicating lower activity levels21. EuroSCORE II, which predicts in-hospital mortality after cardiac surgery, was calculated based on patient-related, cardiac-related,
and operation-related factors22. ETHICAL STATEMENT The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. This study was approved by the Ethics Committee
of our University on 11th July 2022 (the approval number: 22-087-B). The study satisfied the conditions needed to waive the requirement for written informed consent from the study
participants. The ethics committee approved this waiver. STATISTICAL ANALYSIS Continuous variables are described as mean ± standard deviation if the skewness-kurtosis test did not reject the
hypothesis of normality. Otherwise, variables are presented as medians with interquartile range values. Categorical variables are displayed as absolute numbers and percentages. Continuous
variables were assessed using unpaired Student’s t tests or Mann–Whitney U tests, whereas Fisher’s exact test or the chi-squared test was used for categorical variables, as appropriate. Each
cutoff value of TAPSE or RV sphericity index was determined based on the Liu index. The intra- and inter-observer reproducibility for the RV sphericity index were assessed through
Bland–Altman analysis in thirty-four patients, respectively. Before the analysis, the skewness-kurtosis test was conducted to confirm whether the differences followed a normal distribution.
When the RV sphericity index measured by X and Y, 100 (X–Y)/mean (X, Y) is plotted on the y-axis, mean (X, Y) on the x-axis. The risk of two-year MACCE was evaluated using Cox regression
analysis and expressed as hazard ratio (HR) with 95% confidence interval (CI). The follow-up began on the day of TAVR. The follow-up was completed two years after the procedure or the date
when the end-point events were observed, depending on what happened first. Multivariate Cox regression analysis was conducted using forward–backward stepwise selection. Variables with a _p_
value < 0.25 in the univariate Cox regression analysis were selected for the multivariate Cox regression analysis. A minimum of five outcome events per predictor variable was applied to
the construction of multivariate models23. The nonparametric bootstrap method, resampling with replacement 1000 times, was conducted to provide inner validation. Statistical significance was
defined as a _p_ value < 0.05. All statistical analyses were performed using Stata version 14 (StataCorp, College Station, TX, USA). RESULTS PATIENT CHARACTERISTICS After exclusion of 5
patients following TAVR in SAV for AR, this study included a total of 239 patients (age, 86 ± 5 years; males, 32%). HG was present in 161 (67%), classical LFLG in 29 (12%), paradoxical LFLG
in 39 (16%), and NFLG in 10 (4%). Patient characteristics are shown in Table 1. Previous myocardial infarction and concomitant congestive heart failure were observed in 19 (8%) and 122
(51%). CKD stages 3B–5, hepatobiliary system impairment, peripheral vascular disease, cerebrovascular disease, and chronic obstructive pulmonary disease were identified in 91 (38%), 20 (8%),
60 (25%), 29 (12%), and 34 (14%), respectively. EuroSCORE II and clinical frailty score were 3.38 (2.12–4.63) and 4 (3–5). LVEF, LVEDV, and LVESV were 58 ± 11%, 72 (59–94) ml, and 28
(22–43) ml. LVEF of < 40% was observed in 20 patients (8%). TAPSE and SPAP were 20 ± 3 mm and 33 ± 10 mmHg. SPAP was not evaluated in ten patients because of no TR. RV-PA coupling was
0.71 ± 0.31. RV longitudinal diameter, RV mid-ventricular diameter, RV sphericity index, and tricuspid annulus were 65 ± 9 mm, 23 ± 4 mm, 0.36 (0.31–0.39), and 32 ± 5 mm, respectively. PH
based on echocardiography was identified in 58 individuals (25%). MR and TR severity of ≥ 3 + were observed in 3 (1%) and 13 (5%). CLINICAL IMPACT OF THE LEFT AND RIGHT HEART STRUCTURE AND
FUNCTION Two-year MACCE was observed in 34 (14%) and follow-up duration was 556 (220–925) days. Of eligible patients, lost to follow up was observed in 33 patients (14%). Table 2 discloses
the results of univariate Cox regression analysis for two-year MACCE. According to the univariate analysis, both LV and RV parameters predicted the outcomes. However, the multivariate Cox
regression analysis excluded the LV parameter and demonstrated that echocardiographic predictors were TAPSE (adjusted HR in an increase of 1 mm, 0.84; 95% CI, 0.75–0.93; _p_ = 0.001) and RV
sphericity index (adjusted HR in an increase of 0.1, 1.94, 95% CI, 1.17–3.22; _p_ = 0.010) (Fig. 2). Bootstrap method provided the similar results (TAPSE: adjusted HR in an increase of 1 mm,
0.84; 95% CI, 0.75–0.94; _p_ = 0.002; RV sphericity index: adjusted HR in an increase of 0.1, 1.94; 95% CI, 1.12–3.36; _p_ = 0.018). Cutoff values ant the _C_-statistics of TAPSE and RV
sphericity index were 19 mm (0.65 [0.56–0.74]) and 0.377 (0.60 [0.49–0.71]), respectively. Patients with at least an impaired RV parameters underwent a higher incidence of two-year MACCE
compared to those without such disorders (Fig. 3). RA area, SPAP, and TR severity were not correlated with the clinical adverse events. It is noteworthy that RV-PA coupling did not predict
the worse outcomes. PREDICTORS OTHER THAN ECHOCARDIOGRAPHIC PARAMETERS FOR 2-YEAR MACCE As shown in Table 2, BMI, CKD stages 3B–5, hepatobiliary system impairment, concomitant CHF, classical
LFLG, and clinical frailty score of > 4 predicted the clinical adverse events. The multivariate Cox regression analysis identified noncardiac parameters such as CKD stages 3B–5 (adjusted
HR, 2.16; 95% CI, 1.05–4.46; _p_ = 0.036), hepatobiliary system impairment (adjusted HR, 4.74; 95% CI, 1.83–12.32; _p_ = 0.001), male sex (adjusted HR, 2.22; 95% CI, 1.08–4.59; _p_ =
0.031), and clinical frailty score of > 4 (adjusted HR, 2.49; 95% CI, 1.21–5.11; _p_ = 0.013) (Fig. 2). ASSOCIATION BETWEEN THE LEFT AND RIGHT HEART STRUCTURE AND FUNCTION There was a
significant difference of TAPSE between patients with and without reduced LVEF (_p_ = 0.001) (Fig. 4A). PH was not associated with a value of TAPSE (_p_ = 0.624). On the other hand, PH was
significantly related to higher RV sphericity index (_p_ = 0.039) (Fig. 4B) and reduced LVEF was not (_p_ = 0.224). Larger tricuspid annulus diameters and RA areas were observed in patients
with PH (_p_ = 0.002 and _p_ < 0.001, respectively). Association of reduced LVEF with such parameters was not observed (_p_ = 0.642 and _p_ = 0.211, respectively). REPRODUCIBILITY OF THE
RV SPHERICITY INDEX The intra- and inter-observer differences were − 0.01 ± 0.04 and − 0.02 ± 0.07, both of which followed a normal distribution according to the skewness-kurtosis test (_p_
= 0.115 and _p_ = 0.468, respectively). Figure 5 demonstrates good intra- and inter-observer agreement. DISCUSSION The current study demonstrated that RV structural and functional parameters
such as RV sphericity index and TAPSE predicted the subsequent MACCE, whereas those of LV did not. Further, a lower value of TAPSE was associated with reduced LVEF and a higher value of RV
sphericity index was correlated with PH. Noncardiac comorbidities such as impaired kidney function and hepatobiliary system impairment were also clinical adverse predictors. Although severe
AS is the most common left-sided valve lesion, its prognosis can be determined by other lesions. Considering that the RV dysfunction could be a result of advanced LV dysfunction10, early
intervention may be needed before progression of advanced heart failure. Although the RV has been recently regarded as an important predictor in cardiovascular diseases, there are few
investigations evaluating RV structural characteristics, probably due to the complexity. The LV has an ellipsoid-shaped chamber surrounded by relatively thick musculature, whereas the RV has
a crescent-shaped chamber with a thin wall24. Further, multiple interactions between the LV and RV make the interpretation of the RV structural findings much more challenging25. The unique
features make accurate assessment of RV structure difficult; however, some parts of the RV such as shape and tricuspid annulus diameter may reflect its structural disorder to some extent. A
previous single-center study refereed to CT-determined tricuspid annulus as a useful predictor5. Our study evaluated RV sphericity index as well as tricuspid annulus diameter as a marker of
an abnormal RV structure. Consequently, only the former predicted the adverse clinical events. This result would make sense because RV sphericity index evaluates both RV longitudinal and
transverse diameters, whereas tricuspid annulus diameter reflects a transverse diameter. RV sphericity index might reflect RV remodeling considering that PH was associated with higher RV
sphericity index. Association between TAPSE and LV systolic function has been reported previously26. RV dysfunction may be a direct result of LV impairment27, which could be mediated by the
largely septum, but also by LV free wall28. On the other hand, RV dysfunction can also impair LV function by attenuating LV preload and adversely affecting the systolic and diastolic
interaction via the intraventricular septum and the pericardium26. The potential bidirectional influences mentioned above can help explain why the LV has a lesser impact on two-year MACCE
compared to the RV. The clinical impact of the LV features was attenuated in patients with advanced heart failure14. The results of our study recommend assessment of RV characteristics for a
risk stratification. The current study evaluated RV features based on 2-dimensional (2D) images, which may be less accurate than 3-dimensional (3D) images. Indeed, a recent study indicated
a predictive ability of 3D RV ejection fraction was superior to 2D evaluation such as TAPSE and fractional area change29. However, a 3D-image construction of the RV is not available in some
cases because of technical difficulty. Cardiovascular magnetic resonance (CMR) can provide 3D RV images easily and more accurate information regarding structure and function compared to
echocardiography30. It is difficult to apply CMR to all patients scheduled for TAVR in daily clinical practice. Therefore, the RV structure and function should be evaluated by
echocardiography at first to identify patients with obviously normal RV structure and function. It may make sense that CMR is applied for patients in whom echocardiography suggests the RV
impairment or sufficient evaluation is difficult. No association of RV-PA coupling with MACCE could be owing to a higher prevalence of heart failure patients. SPAP might become lower after
removal of afterload mismatch and pulmonary congestion. According to a previous study, SPAP decreased after the procedure, whereas RV systolic function did not significantly improve6.
Another previous study indicated that RV-PA coupling prior to TAVR did not predict long-term mortality31. Noncardiac predictors such as hepatobiliary system impairment and renal impairment
may be modifiable comorbidities in selective patients. However, considering that SPAP was not associated with the clinical adverse events, modifiable parameters did not predict the outcomes
necessarily. It is occasionally difficult to distinguish “true” CKD from cardiorenal syndrome. The same can be said for hepatobiliary system impairment. Future studies dedicated to
investigation of such comorbidities would be needed for expanding understanding of prognosis in patients with severe AS. LIMITATIONS There are several limitations to be addressed. At first,
this was a single-center study. Because of its nature, a selection bias might affect our results. Second, the sample size might not large enough. However, the similar result derived from the
bootstrap method reinforced the reliability of our findings. Third, our study did not include strain analysis, which may contribute to better risk stratification compared to TAPSE or
fractional area change. Finally, as mentioned above, the RV structure and function were not evaluated by CMR. We believe that echocardiographic findings in the present study are reliable;
however, CMR would demonstrate much more accurate findings. CONCLUSIONS The RV sphericity index and TAPSE predicted two-year MACCE, whereas the LV parameters did not. The incidence of the
adverse clinical events was dependent on the RV and noncardiac characteristics. Severe AS is the left-sided heart disease; however, its prognosis is determined by the other lesions. DATA
AVAILABILITY The data supporting the findings of this study will be shared on reasonable request to the corresponding author. REFERENCES * Cribier, A. Invention and uptake of TAVI over the
first 20 years. _Nat. Rev. Cardiol._ 19, 427–428 (2022). Article PubMed Google Scholar * Amabile, N. _et al._ Early and mid-term cardiovascular outcomes following TAVI: Impact of
pre-procedural transvalvular gradient. _Int. J. Cardiol._ 167, 687–692 (2013). Article PubMed Google Scholar * Otto, C. M. _et al._ 2020 ACC/AHA Guideline for the Management of Patients
With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. _Circulation_ 143, e72–e227 (2021).
PubMed Google Scholar * Ghio, S. _et al._ Right heart failure in left heart disease: Imaging, functional, and biochemical aspects of right ventricular dysfunction. _Heart Fail. Rev._ 28,
1009–1022 (2023). Article PubMed Google Scholar * Deseive, S. _et al._ CT-determined tricuspid annular dilatation is associated with increased 2-year mortality in TAVR patients. _JACC
Cardiovasc. Interv._ 13, 2497–2507 (2020). Article PubMed Google Scholar * Lillo, R. _et al._ Right ventricle systolic function and right ventricle-pulmonary artery coupling in patients
with severe aortic stenosis and the early impact of TAVI. _Int. J. Cardiovasc. Imaging_ 38, 1761–1770 (2022). Article PubMed Google Scholar * Adamo, M. _et al._ Prognostic value of right
ventricle to pulmonary artery coupling in transcatheter aortic valve implantation recipients. _J. Cardiovasc. Med. (Hagerstown)_ 23, 615–622 (2022). Article PubMed Google Scholar *
Sultan, I. _et al._ Right ventricle to pulmonary artery coupling in patients undergoing transcatheter aortic valve implantation. _Heart_ 105, 117–121 (2019). Article PubMed Google Scholar
* Généreux, P. _et al._ Staging classification of aortic stenosis based on the extent of cardiac damage. _Eur. Heart J._ 38, 3351–3358 (2017). Article PubMed PubMed Central Google
Scholar * Surkova, E. _et al._ Contraction patterns of the right ventricle associated with different degrees of left ventricular systolic dysfunction. _Circ. Cardiovasc. Imaging_ 14,
e012774 (2021). Article PubMed PubMed Central Google Scholar * Leon, M. B. _et al._ Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery.
_New Engl. J. Med._ 363, 1597–1607 (2010). Article CAS PubMed Google Scholar * Kral Kollars, C. A., Gelehrter, S., Bove, E. L. & Ensing, G. Effects of morphologic left ventricular
pressure on right ventricular geometry and tricuspid valve regurgitation in patients with congenitally corrected transposition of the great arteries. _Am. J. Cardiol._ 105, 735–739 (2010).
Article PubMed Google Scholar * Cameli, M. _et al._ Systematic left ventricular assist device implant eligibility with non-invasive assessment: The SIENA protocol. _J. Cardiovasc.
Ultrasound_ 25, 39–46 (2017). Article PubMed PubMed Central Google Scholar * Higuchi, S. _et al._ Impact of residual mitral regurgitation on survival after transcatheter edge-to-edge
repair for secondary mitral regurgitation. _JACC Cardiovasc. Interv._ 14, 1243–1253 (2021). Article PubMed Google Scholar * Kar, S. _et al._ Relationship between residual mitral
regurgitation and clinical and quality-of-life outcomes after transcatheter and medical treatments in heart failure: COAPT trial. _Circulation_ 144, 426–437 (2021). Article PubMed Google
Scholar * Hahn, R. T. _et al._ Imaging assessment of tricuspid regurgitation severity. _JACC. Cardiovasc. Imaging_ 12, 469–490 (2019). Article PubMed Google Scholar * Lafitte, S. _et
al._ Estimation of pulmonary pressures and diagnosis of pulmonary hypertension by Doppler echocardiography: a retrospective comparison of routine echocardiography and invasive hemodynamics.
_J. Am. Soc. Echocardiogr.: Off. Publ. Am. Soc. Echocardiogr._ 26, 457–463 (2013). Article Google Scholar * Higuchi, S. _et al._ Association of renin-angiotensin system inhibitors with
long-term outcomes in patients with systolic heart failure and moderate-to-severe kidney function impairment. _Eur. J. Internal Med._ 62, 58–66 (2019). Article CAS Google Scholar * Stolz,
L. _et al._ Cardiohepatic Syndrome is associated with poor prognosis in patients undergoing tricuspid transcatheter edge-to-edge valve repair. _JACC Cardiovasc. Interv._ 15, 179–189 (2022).
Article PubMed Google Scholar * Steffen, J. _et al._ TAVI in patients with low-flow low-gradient aortic stenosis-short-term and long-term outcomes. _Clin. Res. Cardiol._ 111, 1325–1335
(2022). Article PubMed PubMed Central Google Scholar * Rockwood, K. & Theou, O. Using the clinical frailty scale in allocating scarce health care resources. _Can. Geriatr. J._ 23,
210–215 (2020). Article PubMed PubMed Central Google Scholar * Nashef, S.A._, et al._ EuroSCORE II. _Eur. J. Cardiothorac. Surg._ 41, 734–744; discussion 744–735 (2012). * Vittinghoff,
E. & McCulloch, C. E. Relaxing the rule of ten events per variable in logistic and Cox regression. _Am. J. Epidemiol._ 165, 710–718 (2007). Article PubMed Google Scholar * Wadghiri,
Y. Z. _et al._ Contrast-enhanced MRI of right ventricular abnormalities in Cx43 mutant mouse embryos. _NMR Biomed._ 20, 366–374 (2007). Article PubMed PubMed Central Google Scholar *
Yamaguchi, S. _et al._ Effect of left ventricular volume on right ventricular end-systolic pressure-volume relation. Resetting of regional preload in right ventricular free wall. _Circul.
Res._ 65, 623–631 (1989). Article CAS Google Scholar * Gupta, S. _et al._ The associations between tricuspid annular plane systolic excursion (TAPSE), ventricular dyssynchrony, and
ventricular interaction in heart failure patients. _Eur. J. Echocardiogr._ 9, 766–771 (2008). Article PubMed PubMed Central Google Scholar * López-Candales, A. _et al._ Right ventricular
systolic function is not the sole determinant of tricuspid annular motion. _Am. J. Cardiol._ 98, 973–977 (2006). Article PubMed Google Scholar * Dong, S. J., Smith, E. R. & Tyberg,
J. V. Changes in the radius of curvature of the ventricular septum at end diastole during pulmonary arterial and aortic constrictions in the dog. _Circulation_ 86, 1280–1290 (1992). Article
CAS PubMed Google Scholar * Orban, M. _et al._ Right ventricular function in transcatheter edge-to-edge tricuspid valve repair. _JACC Cardiovasc. Imaging_ 14, 2477–2479 (2021). Article
PubMed Google Scholar * Nishina, Y. _et al._ Evaluation of right ventricular function on cardiac magnetic resonance imaging and correlation with hemodynamics in patients with chronic
thromboembolic pulmonary hypertension. _Circ. Rep._ 2, 174–181 (2020). Article PubMed PubMed Central Google Scholar * Meucci, M. C. _et al._ Evolution and prognostic impact of right
ventricular-pulmonary artery coupling after transcatheter aortic valve replacement. _JACC Cardiovasc. Interv._ 16, 1612–1621 (2023). Article PubMed Google Scholar Download references
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division of Cardiology, Department of Medicine, Showa University School of Medicine, Tokyo, Japan Satoshi Higuchi, Yasuhide Mochizuki, Hidenari
Matsumoto & Toshiro Shinke * Division of Cardiovascular Surgery, Department of Surgery, Showa University School of Medicine, Tokyo, Japan Tadashi Omoto, Tomoaki Masuda, Kazuto Maruta
& Atsushi Aoki Authors * Satoshi Higuchi View author publications You can also search for this author inPubMed Google Scholar * Yasuhide Mochizuki View author publications You can also
search for this author inPubMed Google Scholar * Tadashi Omoto View author publications You can also search for this author inPubMed Google Scholar * Hidenari Matsumoto View author
publications You can also search for this author inPubMed Google Scholar * Tomoaki Masuda View author publications You can also search for this author inPubMed Google Scholar * Kazuto Maruta
View author publications You can also search for this author inPubMed Google Scholar * Atsushi Aoki View author publications You can also search for this author inPubMed Google Scholar *
Toshiro Shinke View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conceptualization, S.H.; formal analysis, S.H. and H.M.; data curation and
investigation, S.H., Y.M., T.O., T.M., K.M., A.A., and T.S.; writing—original draft preparation, S.H.; writing—review and editing, Y.M., T.O., H.M., and T.S. All authors have read and agreed
to the published version of the manuscript. CORRESPONDING AUTHOR Correspondence to Satoshi Higuchi. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS
OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or
other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not
included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission
directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Higuchi,
S., Mochizuki, Y., Omoto, T. _et al._ Clinical impact of the right ventricular impairment in patients following transcatheter aortic valve replacement. _Sci Rep_ 14, 1776 (2024).
https://doi.org/10.1038/s41598-024-52242-w Download citation * Received: 15 November 2023 * Accepted: 16 January 2024 * Published: 20 January 2024 * DOI:
https://doi.org/10.1038/s41598-024-52242-w SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative